Omeprazole sodium freeze-dried lipidosome preparation and preparation method thereof
A technology of omeprazole sodium and liposome preparation, which is applied in the field of freeze-dried liposome preparation of omeprazole sodium and its preparation, can solve problems such as poor stability, achieve stable product quality, improve therapeutic index, The effect of convenient clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
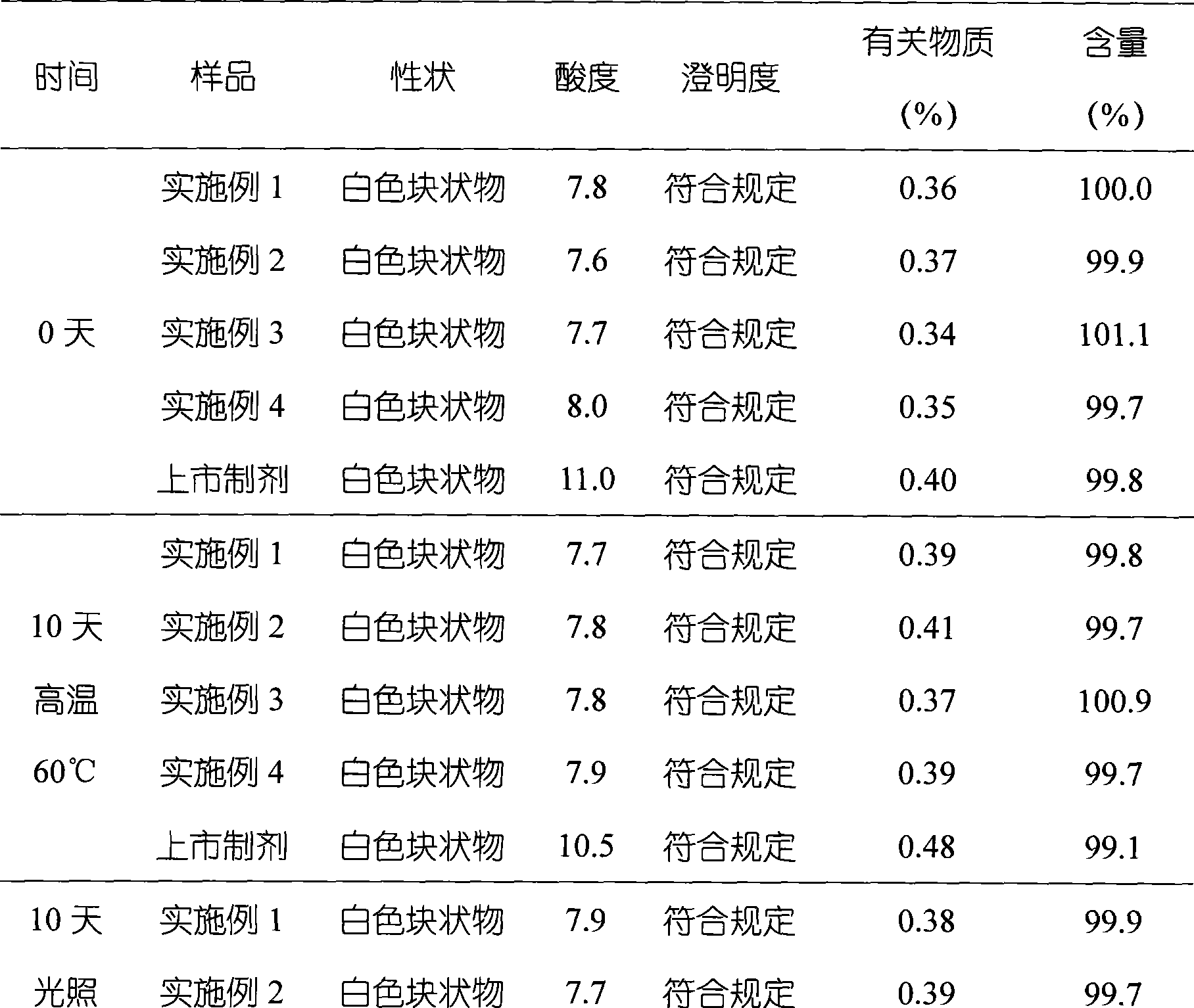
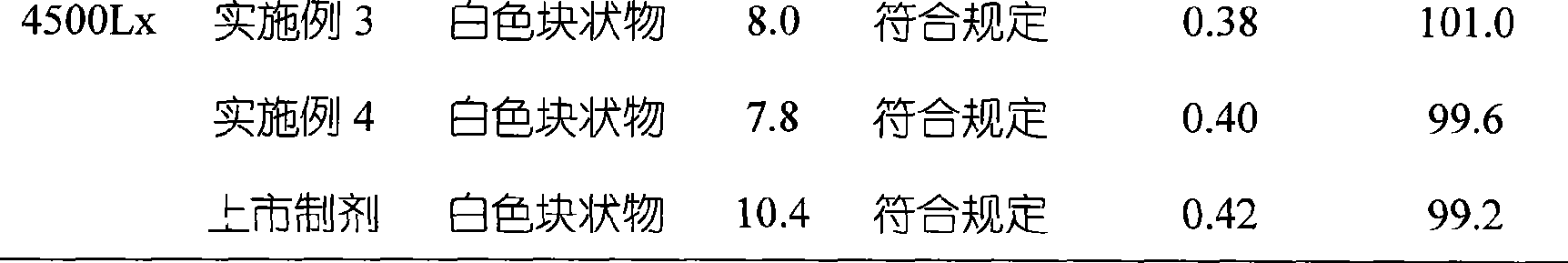
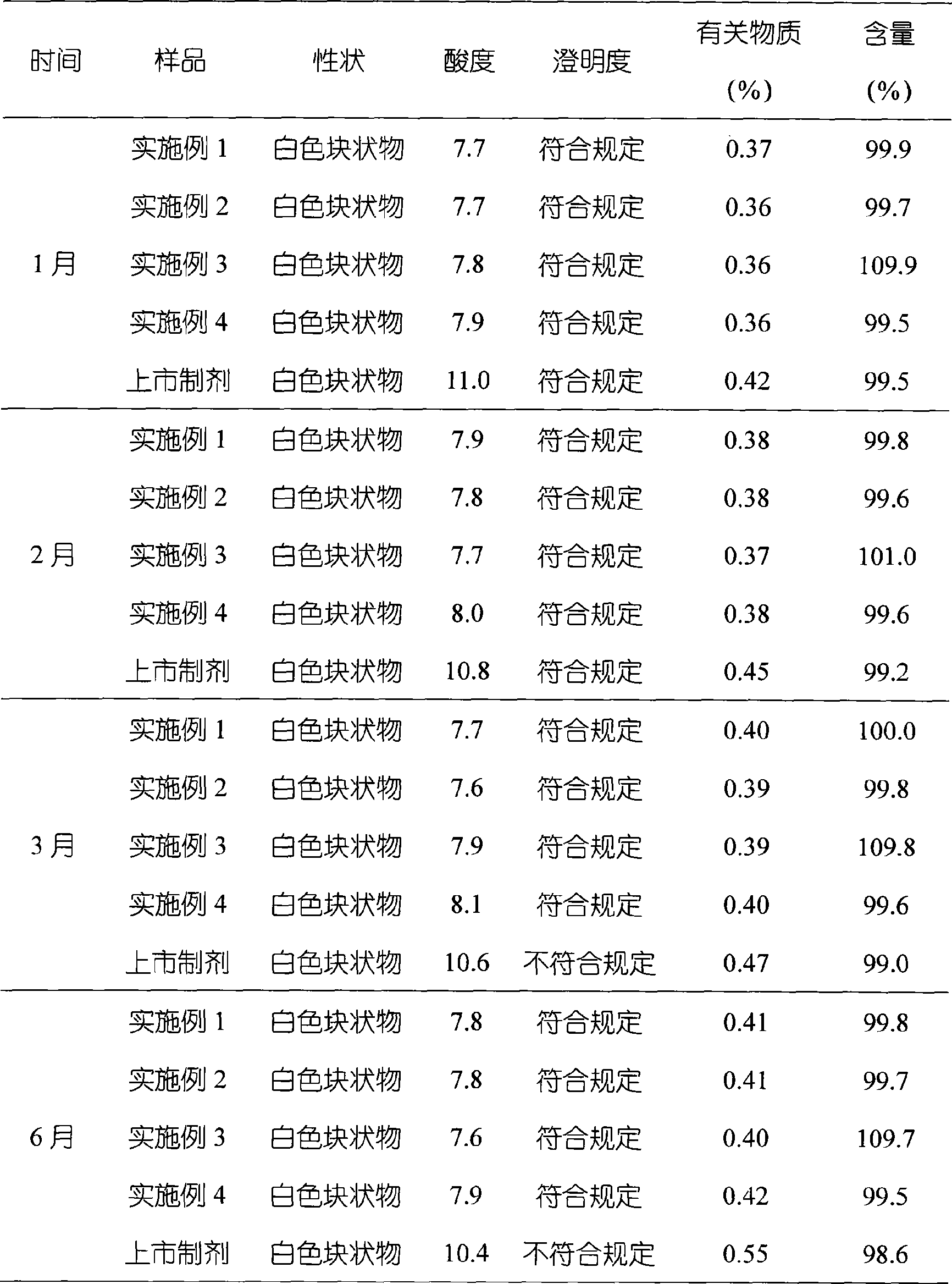
Embodiment 1
[0040] Embodiment 1 The preparation of omeprazole sodium freeze-dried liposome preparation
[0041] Prescription (1000 bottles): Omeprazole Sodium 40g
[0042] Soy Lecithin 75g
[0043] Cholesterol 20g
[0044] Vitamin E 10g
[0045] Mannitol 40g
[0046] Preparation Process:
[0047] (1) Weigh 75g of soybean lecithin, 20g of cholesterol and 10g of vitamin E and dissolve them in 800ml of ethanol, heat and melt, add a phosphate buffer solution with a pH value of 7.8, heat to remove ethanol, transfer to a tissue masher, and stir at a high speed for 10 minutes, filter, sterilize, and sonicate for 10 minutes;
[0048] (2) In a sterile room, under 100-grade conditions, add 40 g of sterilized omeprazole sodium and 40 g of mannitol to dissolve, filter, and fill in vials;
[0049] (3) Pre-freeze at -35°C for 3 hours, then dry under reduced pressure and vacuum at -45°C to -5°C for 25 hours, and finally dry at 35°C for 5 hour...
Embodiment 2
[0051] Embodiment 2 The preparation of omeprazole sodium freeze-dried liposome preparation
[0052] Prescription (1000 bottles): Omeprazole Sodium 20g
[0054] Cholesterol 5g
[0056] Mannitol 15g
[0057] Lactose 30g
[0058] Preparation Process:
[0059] (1) Take by weighing 20g egg yolk lecithin, 5g cholesterol and sodium bisulfite 7.5g and dissolve in the mixture of 500ml isopropanol and ethanol with a volume ratio of 5:1, heat and melt, add pH value 7.8 citrate buffer solution, Heat to evaporate isopropanol and ethanol, transfer to a tissue masher, stir at high speed for 30 minutes, filter, sterilize, and sonicate for 20 minutes;
[0060] (2) In a sterile room, under 100-grade conditions, add 20 g of sterilized omeprazole sodium, 15 g of mannitol and 30 g of lactose to dissolve, filter, and fill in vials;
[0061] (3) Pre-freeze at -45...
Embodiment 3
[0063] Embodiment 3 The preparation of omeprazole sodium freeze-dried liposome preparation
[0064] Prescription (1000 bottles): Omeprazole Sodium 20g
[0065] Hydrogenated egg yolk lecithin 10g
[0066] Ascorbyl Palmitate 15g
[0067] Sorbitol 10g
[0068] Trehalose 15g
[0069] Preparation Process:
[0070] (1) Weigh 10g of hydrogenated egg yolk lecithin and 15g of ascorbyl palmitate, dissolve them in 500ml of isopropanol and ethanol with a volume ratio of 5:1, heat and melt, add a pH value of 7.8 phosphate buffer solution, heat and evaporate to remove isopropanol Propanol and ethanol, transferred to a tissue masher, stirred at high speed for 20 minutes, filtered, sterilized, and ultrasonicated for 10 minutes;
[0071] (2) In a sterile room, under 100-grade conditions, add 20 g of sterilized omeprazole sodium, 10 g of sorbitol and 15 g of trehalose and dissolve, filter, and fill in vials;
[0072] (3) Pre-freeze a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com