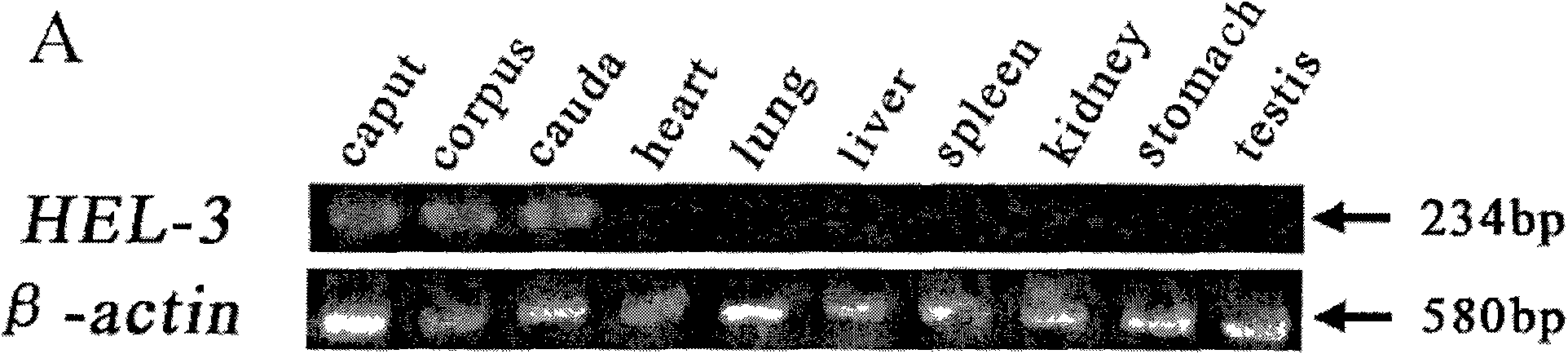Preparation and application of sperm binding protein HEL-127 specifically expressed in human epididymis
A technology of HEL-127 and protein, which is applied in the fields of biotechnology and medicine, and can solve problems such as no reports and no homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Tissue-specific RT-PCR analysis
[0040] Trizol reagent was used to extract the total RNA of human heart, lung, liver, spleen, kidney, stomach, testis, head of epididymis, body of epididymis and tail of epididymis, the quality of total RNA was identified by agarose gel electrophoresis, and RNA was quantified by UV spectrophotometer. Then reverse transcription (RT) was performed. cDNA synthesis system: primers (oligo d T 18, 25pmol / mL) 3ul, total RNA (300ng / ul) 3ul, ddH 2O (DEPC treatment) 4ul, total volume 10ul; denature at 65°C for 5min, quickly place on ice, then add the following components: 5×AMV buffer 4ul, 10mmol / L dNTP mix 4uL, AMV 1ul, ddH 2 O (DEPC treatment) has a total volume of 20ul; extended at 42°C for 1h, incubated at 70°C for 10min to terminate the reaction, and stored at -20°C for later use.
[0041] The reversed cDNA template was amplified by PCR to detect tissue-specific expression of the gene. The primer sequence used: upstream: 5'-TTGG...
Embodiment 2
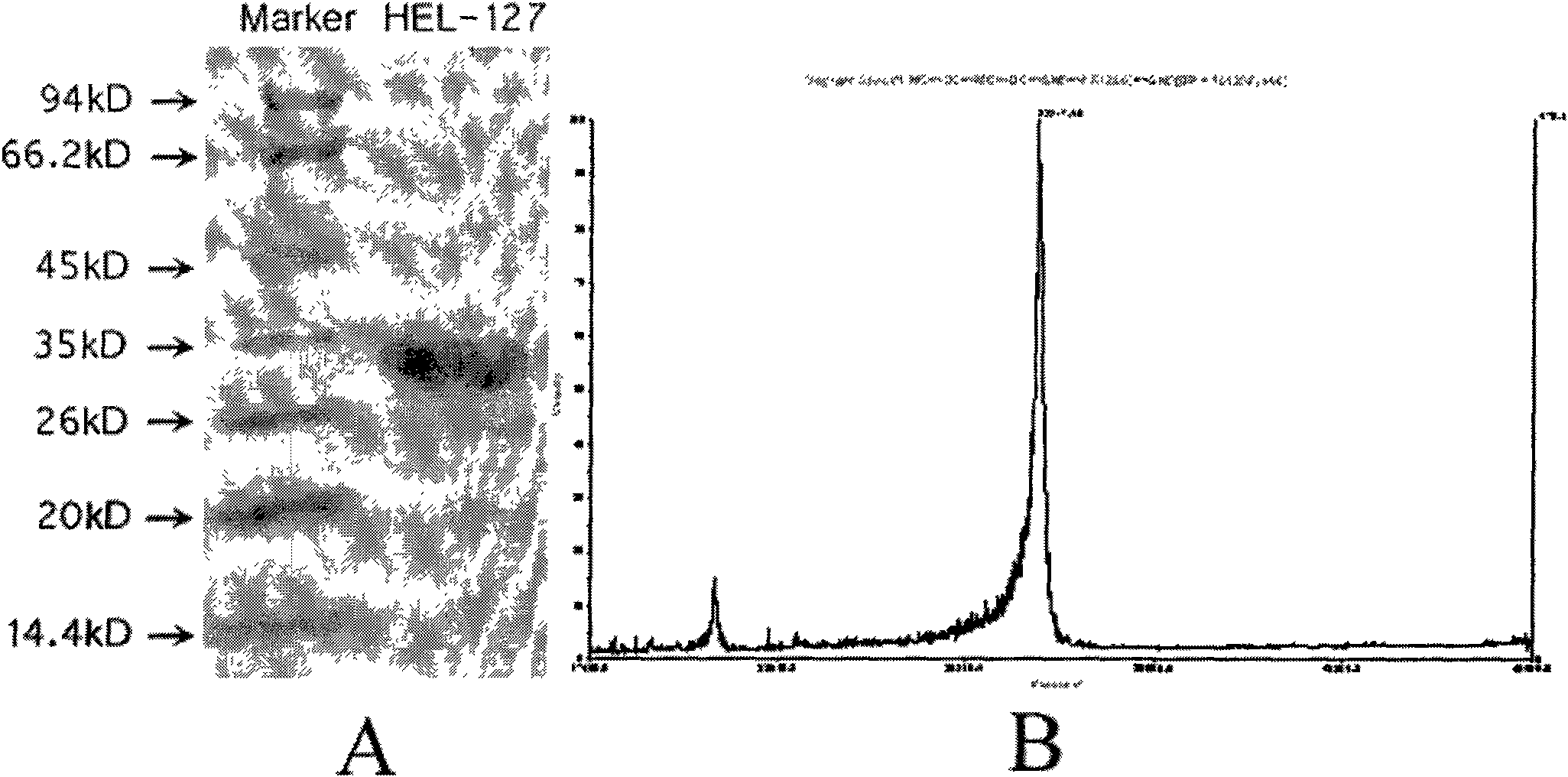
[0042] Example 2HEL-127 expression vector construction, protein purification and molecular weight identification
[0043] According to the gene sequence of HEL-127, synthesize a pair of specific primers for amplifying the mature coding region: upstream SC112-F: 5'-TT GGTACC GACGACGACGACAAGCAGTGCATAACATGCCACC-3', downstream SC112-R: 5'-GGC GAATTC TCAGAGTTTAAAGTTACAGTAATTGTAG-3, the upstream and downstream endonuclease sites KpnI and EcoRI were introduced respectively. Using the human epididymis cDNA library as a template, the target gene was directly amplified by PCR, and then cloned into the cloning vector pGM-T vector for sequencing identification. The SC112 gene identified by sequencing was cloned into the expression vector pET32b(+) through KpnI and EcoRI sites, making it consistent with the reading frame of the fusion tag. The recombinant expression vector pET32b(+)-sc112 was transferred into E.coliBL21(DE3) competent cells, and the engineered strain obtained was induce...
Embodiment 3
[0045] Embodiment 3 HEL-127 polyantiserum preparation
[0046] The purified recombinant protein was used to immunize Kunming white mice, and SC112 protein was fully mixed with Freund's complete adjuvant at a dose of 26ug for each mouse, and injected intramuscularly at multiple points on the back; Immunization was boosted three times with incomplete adjuvant; 7 days later, blood was collected from the orbit, and ELLSA was used to determine the titer, and then the blood was collected from the tibia, and the antiserum was separated and stored at -70°C for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com