Conjugated polymer material of fluorine, pyrene and perylene and preparation thereof
A technology of conjugated polymers and polymers, applied in the field of conjugated polymer materials and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
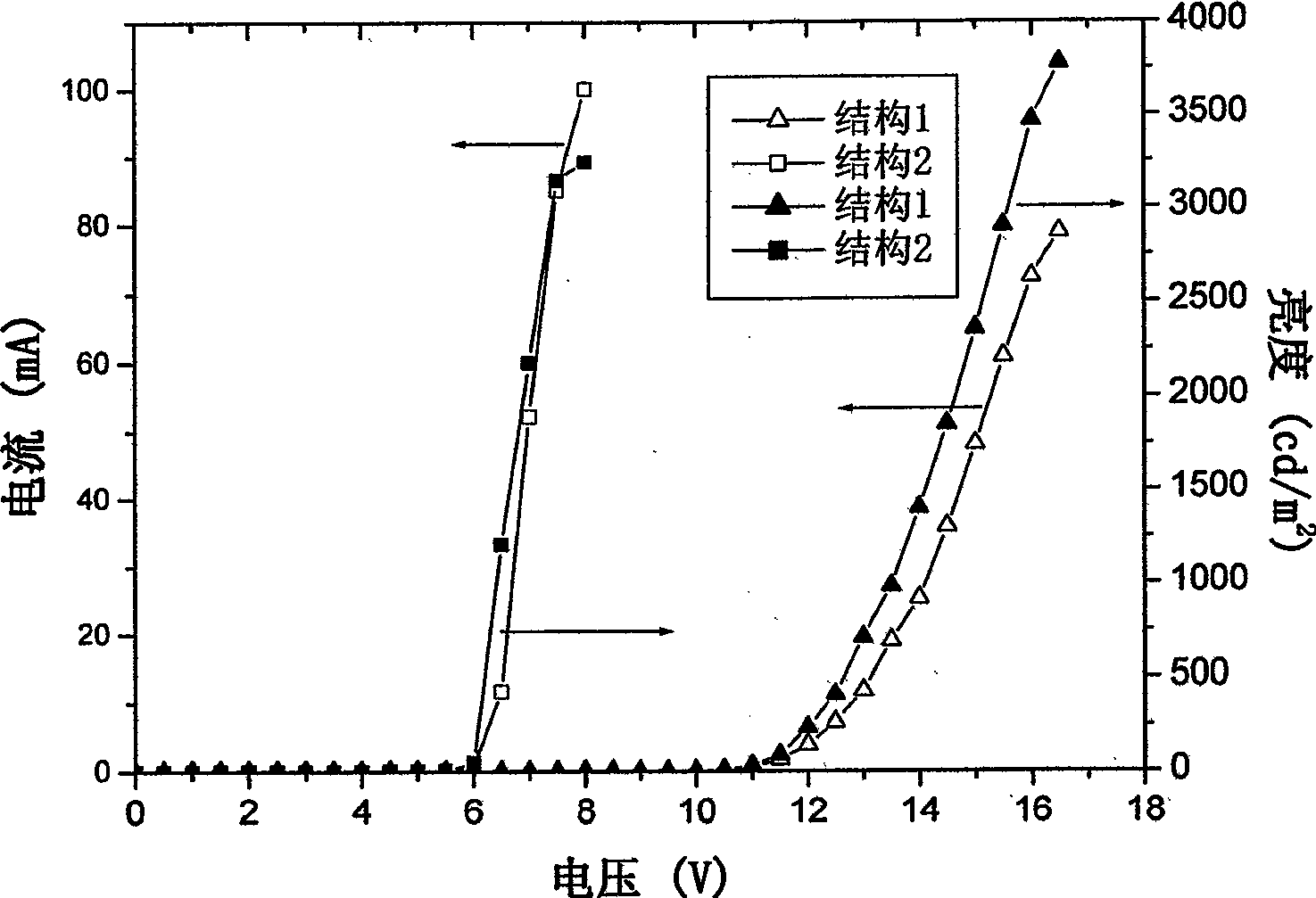
Examples
Embodiment 1
[0086] Example 1: Synthesis of polymer structure I1B (m=1, wherein the alkyl group is n-octyl)
[0087] Poly[(9,9-di-n-octylfluorene-2,7-diyl)-alt-co-(pyrene-1,6(8)-diyl)] (PDOFP). 2,7-dibromo-9,9-dioctylfluorene (M1) (5.4844g, 1.0mmol), 1,6(8)-dibromopyrene borate (PB2) (0.3700g, 1.0mmol) placed In the 100mL flask with a stirring bar in advance, vacuumize and pass nitrogen three times, inject oxygen-free toluene (20mL) into the flask with a syringe. The mixture was heated to 80°C and stirred until the monomers were completely dissolved, and tetrakis(triphenylphosphide)palladium (Pd(PPh 3 ) 4 ) (0.01 mmol) in toluene (10 mL), and inject anaerobic nitrogen-saturated 2M sodium carbonate solution (5 mL) with a syringe. Then the system was heated to 90°C, and after 48 hours of reaction, phenylboronic acid (1.2g, 0.1mmol) was added to react for 12h to seal off the bromine end of the copolymer, and then bromobenzene (1mL, 1.5g, 10mmol) was added to react for 12h to seal off the c...
Embodiment 2
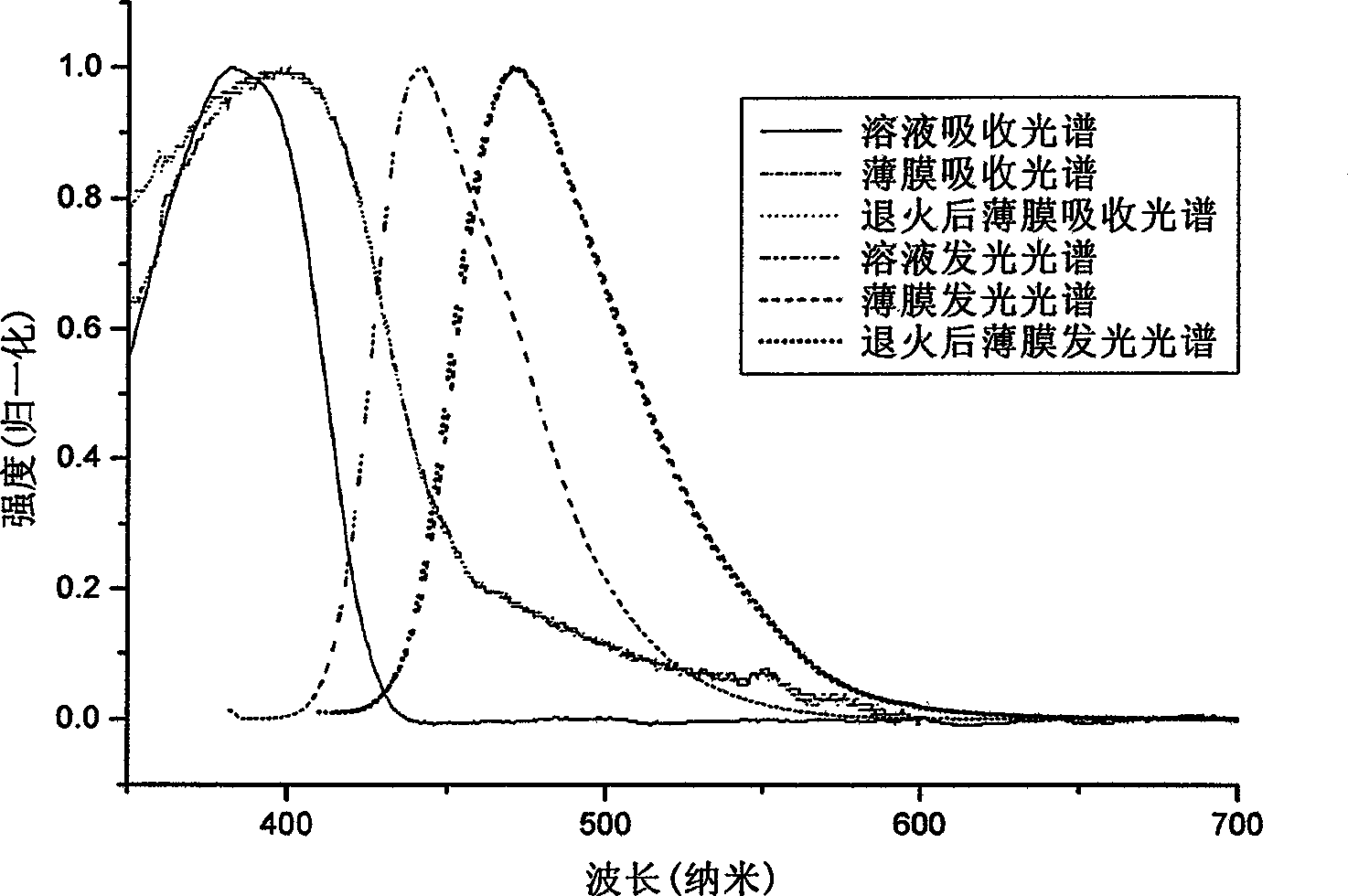
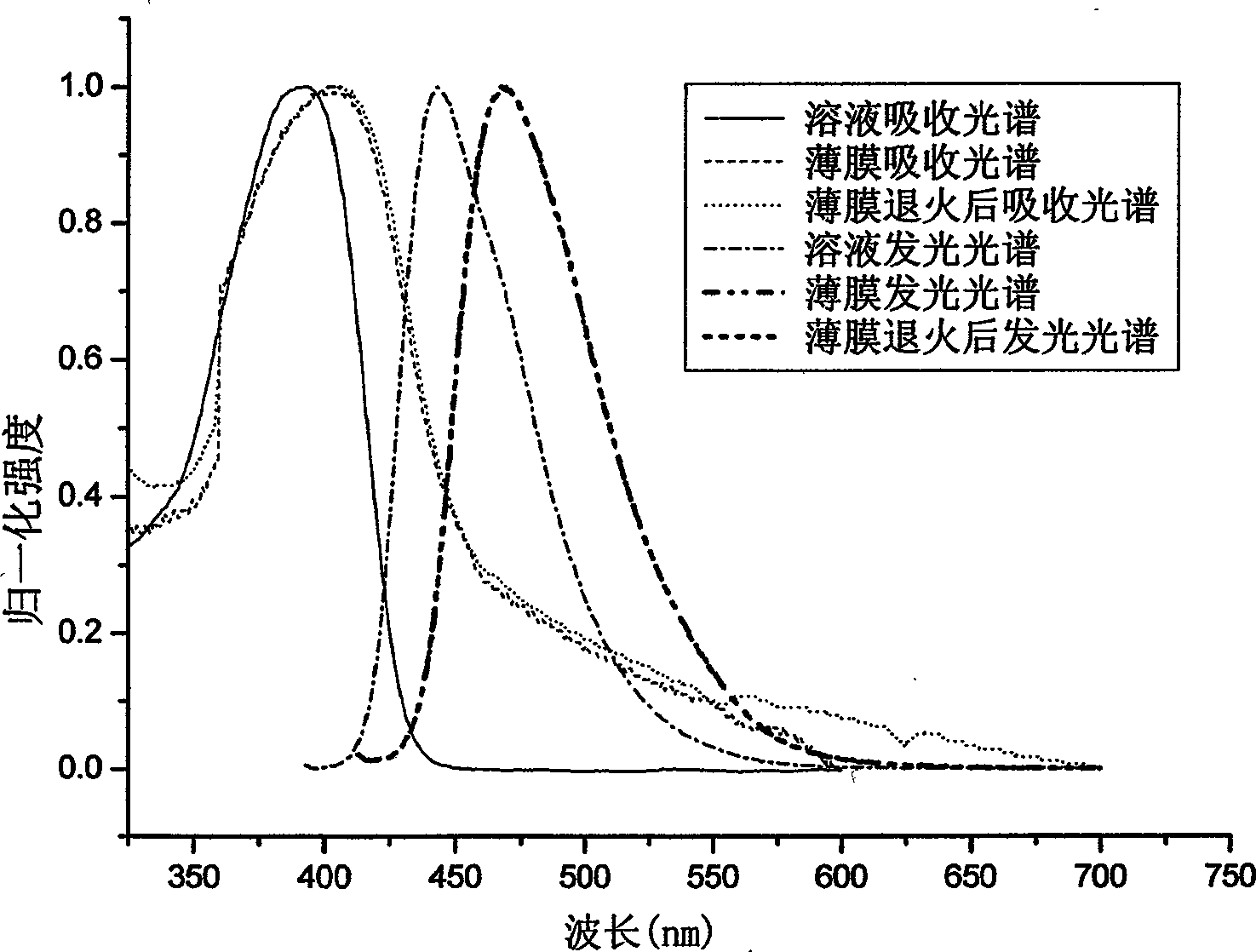
[0093] Example 2: Absorption spectra of solutions and thin films of the polymer PDOFP (product of Example 1), photoluminescence spectrometry.
[0094] Dissolve PDOFP in THF solution, and use Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer to measure the absorption and emission spectra. The photoluminescence spectrum is excited by the maximum absorption wavelength of ultraviolet absorption. The solid film is formed by dropping the solution on a transparent glass plate after the solvent evaporates. The fluorescence quantum efficiency of the solution is measured by 10 in cyclohexane -6 The 9,10-dibenzanthracene solution of M (with a quantum efficiency of 0.9) was measured as a standard, and the measured value was 76%.
[0095] In the wavelength range greater than 300nm, the PDOFP solution has a maximum absorption peak of 383nm, the absorption peak is relatively smooth, and the maximum emission of the photoluminescence spectrum is 442nm....
Embodiment 3
[0097] Embodiment 3: the synthesis of polymer structure I 1A (wherein alkyl is n-octyl)
[0098] Poly[(9,9-di-n-octylfluorene-2,7-diyl)-alt-co-(pyrene-2,7-diyl)]. 2,7-dibromo-9,9-dioctylfluorene (M1) (5.4844g, 1.0mmol), 2,7-dibromopyrene borate (PB1) (0.3700g, 1.0mmol), tetrakis (tri Phenylphosphide) palladium (Pd(PPh 3 ) 4 ) (0.01mmol) in toluene (10mL), according to the similar preparation method as Hecheng PDOFP, a light yellow solid (0.58g, 70%) can be obtained. Anal. Calcd. C, 91.78; H, 8.22. found C, 91.52; H, 8.27. The polymer was dissolved in THF, and the molecular weight of the polymer was determined by gel chromatography (THF was the eluent). The number average molecular weight (Mn) was 49869, the weight average molecular weight (Mw) was 81286, and the polydispersity coefficients were 1.63, respectively. The difference between polymer I1A and polymer I1B is that in the former, pyrene is connected to fluorene through 2,7, while in the latter, the connection includ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com