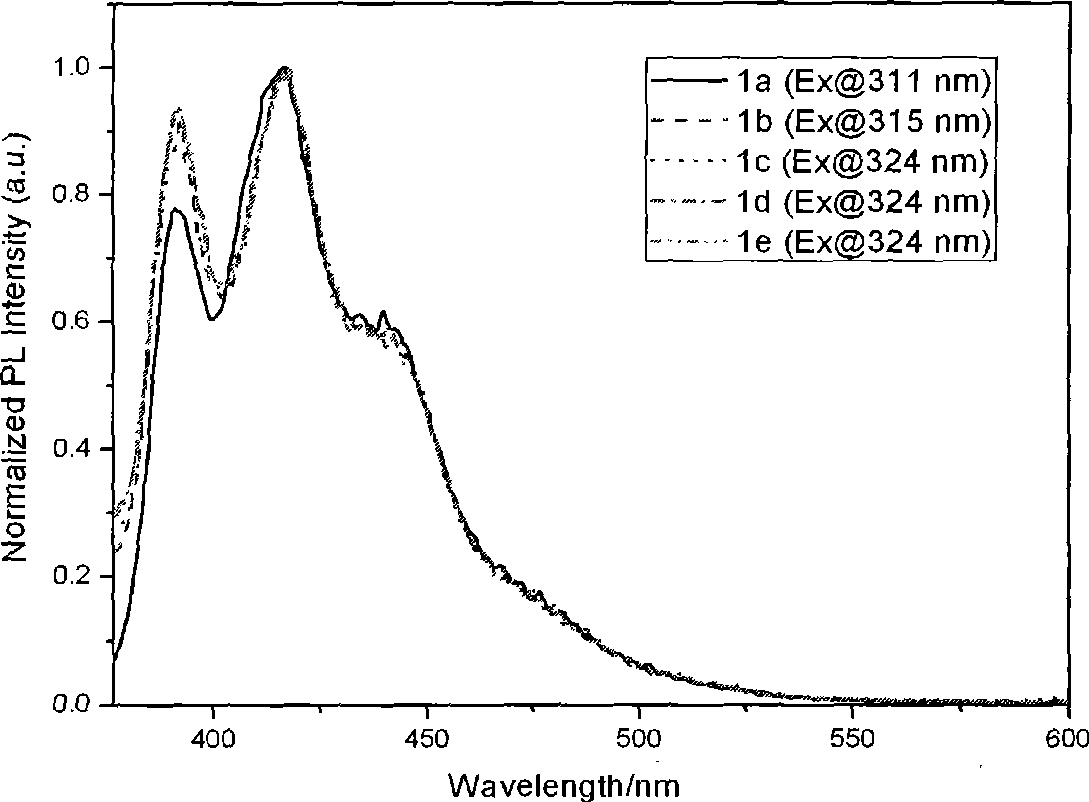
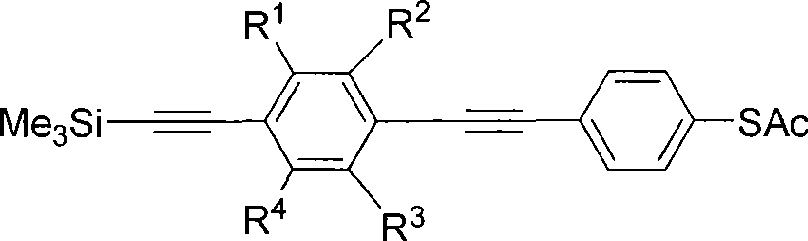
Organic luminescent material phenylene ethynylene dimmer, synthesizing method and use
A technology of phenylene acetylene and synthesis method, which is applied in the direction of light-emitting materials, silicon organic compounds, chemical instruments and methods, etc., and can solve the problems of limited research on the types, properties and photoelectric properties of alkyl substituents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: 4-(2-methyl-4-trimethylsilylethynyl phenylethynyl) phenyl thioacetate ( 1a )Synthesis
[0052] Add 1.89 g (6.0 mmol) of 2-methyl-4-trimethylsilylethynyl iodobenzene, 0.21 g (0.30 mmol) of bistriphenylphosphine palladium dichloride, and 0.057 g (0.30 mmol) of copper iodide into a 100 mL four-necked flask. g (0.30mmol), 4.48g (10.8mmol) of tri-n-butylamine and 30mL tetrahydrofuran, stirred at room temperature for 0.5h under nitrogen protection, then added 1.16g (6.6mmol) of p-thioacetylphenylacetylene, and continued the reaction for 24h before adding The reaction was quenched with 50 mL of water. Dichloromethane (30mL × 3) was extracted, the organic layer was washed with saturated NaCl solution, anhydrous MgSO 4 Dry, evaporate the solvent under reduced pressure to obtain a dark brown solid, which is separated and purified by column chromatography (silica gel filling, n-hexane / ethyl acetate) to obtain a light yellow solid 4-(2-methyl-4-trimethylsilylethynyl...
Embodiment 2
[0053] Embodiment 2: 4-(2-ethyl-4-trimethylsilylethynyl phenylethynyl) phenyl thioacetate ( 1b )Synthesis
[0054] Add 1.97 g (6.0 mmol) of 2-ethyl-4-trimethylsilylethynyl iodobenzene, 0.21 g (0.30 mmol) of bistriphenylphosphine palladium dichloride, and 0.057 g (0.30 mmol) of copper iodide into a 100 mL four-neck flask. g (0.30mmol), 4.48g (10.8mmol) of tri-n-butylamine and 30mL tetrahydrofuran, stirred at room temperature for 0.5h under nitrogen protection, then added 1.16g (6.6mmol) of p-thioacetylphenylacetylene, and continued the reaction for 24h before adding The reaction was quenched with 50 mL of water. Dichloromethane (30mL × 3) was extracted, the organic layer was washed with saturated NaCl solution, anhydrous MgSO 4 Dry, evaporate the solvent under reduced pressure to obtain a black solid, which is separated and purified by column chromatography (silica gel filling, n-hexane / ethyl acetate) to obtain a light yellow solid 4-(2-ethyl-4-trimethylsilylethynyl- Phenyle...
Embodiment 3
[0055] Embodiment 3: 4-(2,6-dimethyl-4-trimethylsilylethynyl phenylethynyl) phenyl thioacetate ( 1c )Synthesis
[0056] Add 1.97g (6.0mmol) of 2,6-dimethyl-4-trimethylsilylethynyl iodobenzene into a 100mL four-neck flask, 0.21g (0.30mmol) of bistriphenylphosphine palladium dichloride, and iodide Cuprous 0.057g (0.30mmol), tri-n-butylamine 4.48g (10.8mmol) and 30mL tetrahydrofuran, stirred at room temperature for 0.5h under nitrogen protection, then added 1.16g (6.6mmol) of p-thioacetylphenylacetylene, and continued the reaction After 30 h, 50 mL of water was added to quench the reaction. Dichloromethane (30mL × 3) was extracted, the organic layer was washed with saturated NaCl solution, anhydrous MgSO 4 Dry, and evaporate the solvent under reduced pressure to obtain a black solid, which is separated and purified by column chromatography (filling with silica gel, n-hexane / ethyl acetate) to obtain a light yellow solid 4-(2,6-dimethyl-4-trimethylsilane Ethynyl-phenylethynyl)ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com