Huperzine A double-layer osmotic pump controlled release tablets and preparation method thereof
An osmotic pump controlled release and huperzine A technology is applied in the field of Huperzine A double-layer osmotic pump controlled release tablet and its preparation, which can solve the problems of pain, too fast drug release, complicated preparation process and the like, and achieves convenient application. , The effect of stable blood concentration and reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
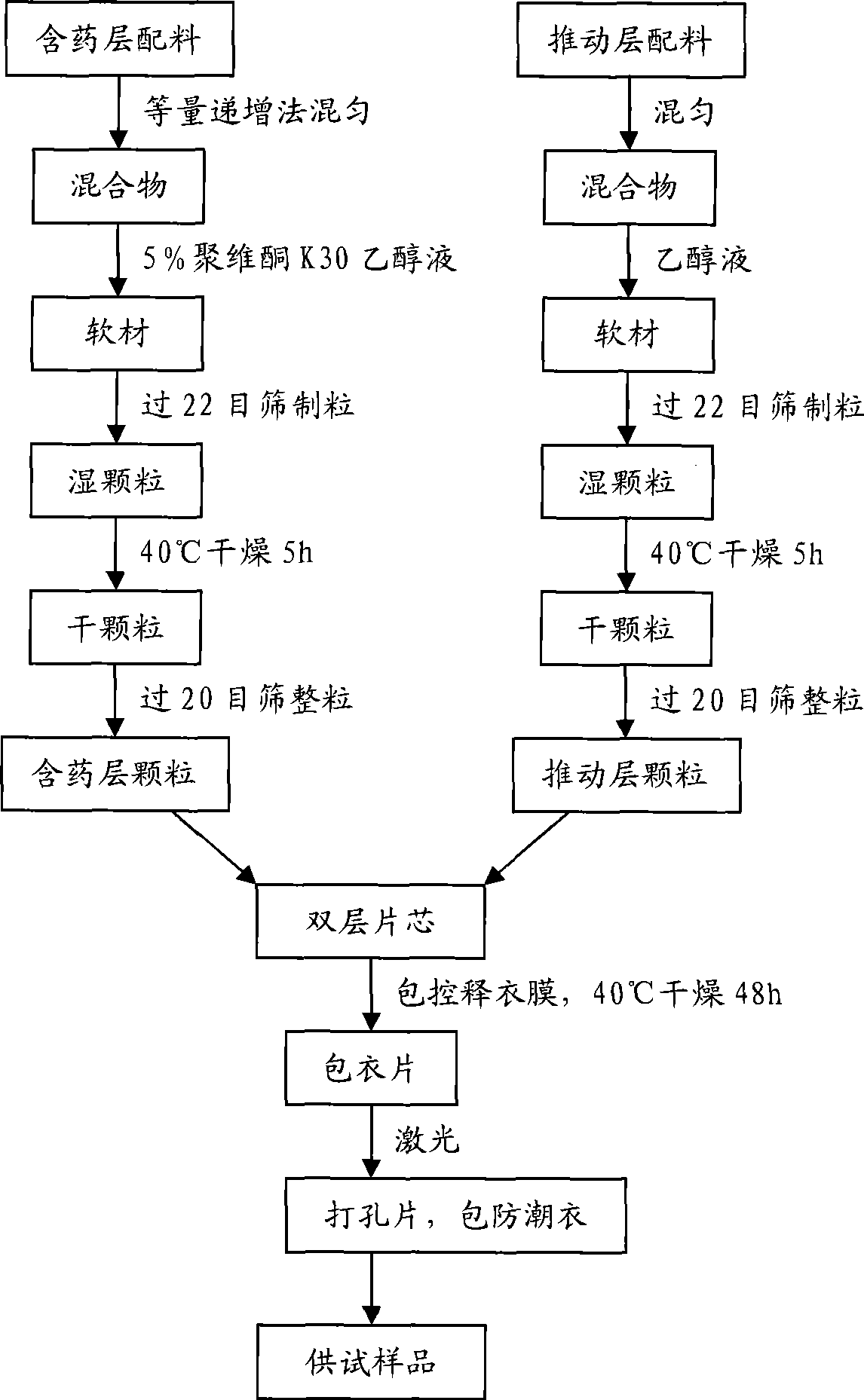
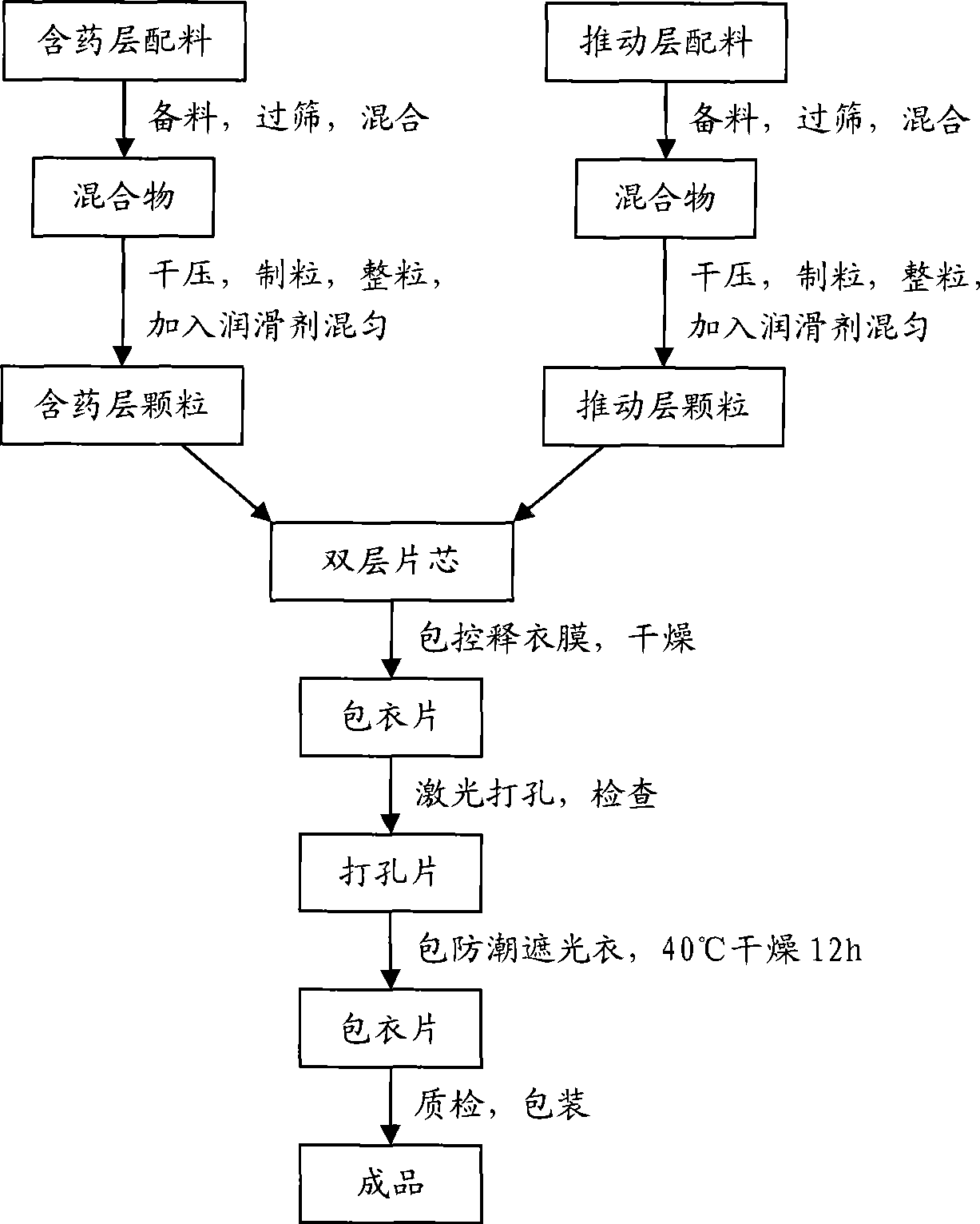
Image
Examples
Embodiment 1
[0080] prescription:
[0081]
[0082] Preparation process: a. Pass huperzine A raw material, red iron oxide, and yellow iron oxide through 80-mesh sieve respectively, and pass the remaining auxiliary materials through 60-mesh sieve respectively. The other medicinal materials are mixed through a 60-mesh sieve, and then the mixture is gradually mixed with the main drug and pigment, and placed in a mixer for mixing;
[0083] b. The obtained mixed powder is dry-pressed into large tablets, the set pressure is 6.0-6.5MPa, the feed speed is 2rpm, the roller shaft speed is 2.3-2.5rpm, and the particles between 20-60 meshes are sieved;
[0084] c. Compress the double-layer tablet core, set the pressure to 4.5MPa;
[0085] d. Prepare the controlled-release coating solution to completely dissolve and clarify, and set aside;
[0086] e. The tablet core is coated with a controlled-release coating film, set the tablet bed weight to 5.0Kg, the rotation speed to 10rpm, the inlet air tem...
Embodiment 2
[0091] prescription:
[0092]
[0093] Preparation process: with embodiment 1. The weight gain of coating film during coating is: 20mg.
Embodiment 3
[0095] prescription:
[0096]
[0097]
[0098] Preparation process: with embodiment 1. The weight gain of coating film during coating is: 26mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com