Chloromycetin eye drops containing sulfobutyl-beta-cyclodextrins and preparation method thereof
A technology containing sulfobutyl ether and sulfobutyl ether, applied in the field of medicine, can solve the problems of reducing the dissolution rate, affecting the compliance of patients with drugs, etc., and achieving the effect of improving stability
Inactive Publication Date: 2009-06-17
SHENYANG PHARMA UNIVERSITY
View PDF1 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, because chloramphenicol is slightly soluble in water, especially adding substances such as sodium hyaluronate to increase the viscosity of the solution to improve bioavailability, the dissolution rate will decrease
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
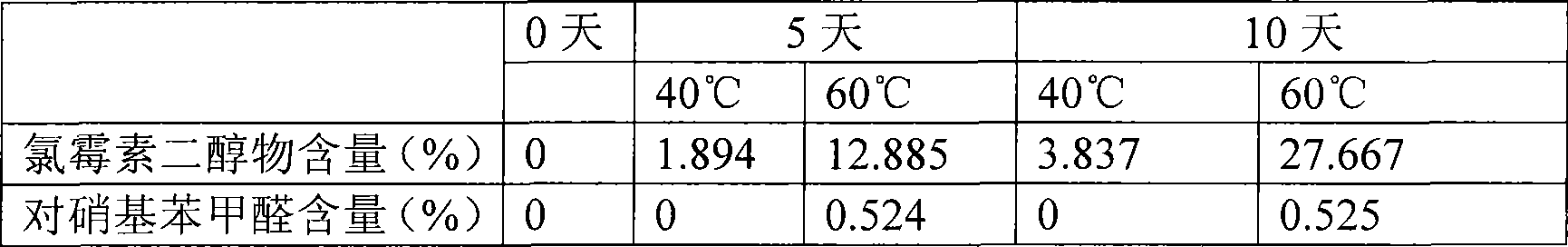
[0027] Take 0.25g of chloramphenicol, 1.2028g of boric acid and 0.0573g of borax, and add water to 100ml.
Embodiment 2
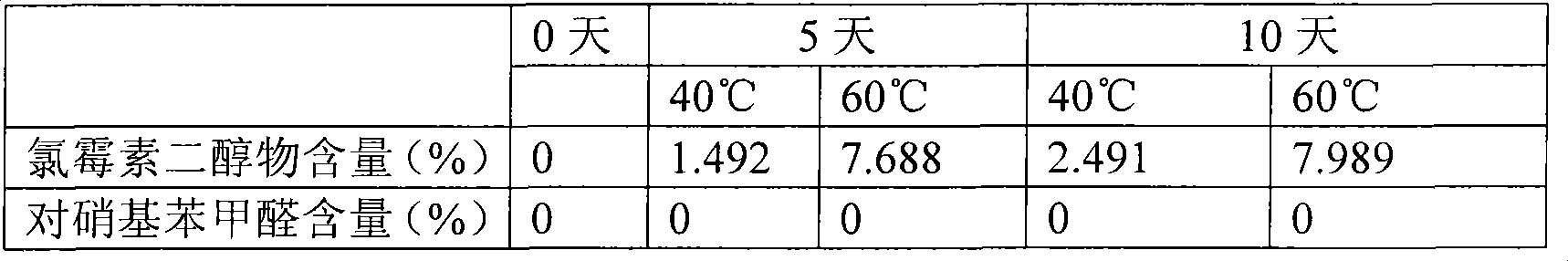
[0029] Take 0.25g of chloramphenicol, 1.2028g of boric acid, 0.0573g of borax, and add water to 100ml of SBE-β-CD1g.
Embodiment 3
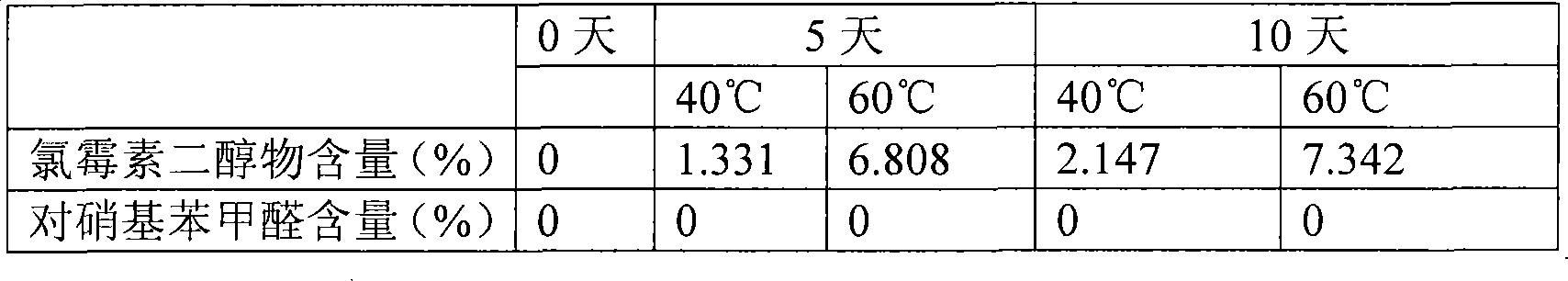
[0031] Take 0.25g of chloramphenicol, 1.2028g of boric acid, 0.0573g of borax, and 5g of SBE-β-CD5g and add water to 100ml.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The present invention belongs to technical field of medicine, and relates to a chloromycetin eyedrop comprising sulfobutyl ether-beta-cyclodextrin (SBE-beta-CD) and a preparing method thereof. The chloromycetin eyedrop has excellent stability. The concentration of sulfobutyl ether-beta-cyclodextrin is 5%-10%. The preparing method comprises the following steps: weighting SBE-beta-CD, adding into boiled injection water and mixing, placing to cold after totally dissolving and adding pH modifying agent into the preparation, then adding an unimycetin with a recipe amount, heating and dissolving in a temperature below 60 DEG C, adding injection water to total amount, and filtering with a millipore filter of 0.22 mu m. The obtained preparation is embedded in the ampoule after checking to be qualified. The chloromycetin eyedrop which adopts SBE-beta-CD as packaging material has the advantages of reducing the decomposition under the conditions of light and heat, retaining the bioactivity, reducing the excitation to eye and prolonging the effective period of chloromycetin eyedrop.
Description
Technical field: [0001] The invention belongs to medical technology, and relates to a chloramphenicol eye drop and a preparation method thereof, in particular to a chloramphenicol eye drop containing sulfobutyl ether-β-cyclodextrin (SBE-β-CD) and a preparation thereof method. Background technique: [0002] Chloramphenicol eye drops, a common broad-spectrum antibiotic eye drops, is a colorless or almost colorless clear liquid. For the treatment of eye infections caused by Escherichia coli, Haemophilus influenzae, Klebsiella, Staphylococcus aureus, hemolytic streptococcus and other sensitive bacteria, such as trachoma, conjunctivitis, keratitis, blepharitis, etc. . Chloramphenicol is relatively stable in neutral and weakly acidic solutions, but light, heat, and the pH of the medium all affect its stability. During production and storage, it is hydrolyzed to produce 1-(4-nitrophenyl)-2-amino -1,3-propanediol (referred to as diol), which reduces the antibacterial activity. T...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/08A61K47/48A61K31/165A61K47/40A61P31/04A61P27/02
Inventor 何仲贵王新旭王永军孙进王安娜
Owner SHENYANG PHARMA UNIVERSITY
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com