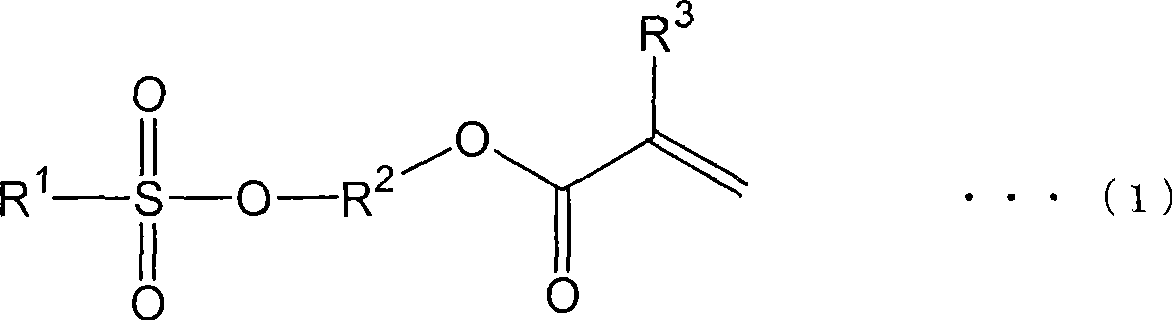
Method for producing (meth)acrylate and (meth)acrylate composition
A technology for acrylates and manufacturing methods, which is applied in the direction of carboxylate preparation, organic chemical methods, chemical instruments and methods, etc., can solve the problems of insufficient storage stability and thermal stability of acrylates, and achieve improved storage stability and Thermal stability, effect of suppressing acid value increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0269] Examples and comparative examples are described below to further describe the present invention in detail.
[0270] [various analysis methods]
[0271] ○ acid value
[0272] According to JIS K-0070-1992, the obtained acrylate was dissolved in ethanol and titrated with potassium hydroxide solution using phenolphthalein as an indicator. The acid value of the sample was calculated from the following formula.
[0273] Acid value [mg—KOH / g]=N×T×f×56.11 / W
[0274] N: Concentration of alcoholic potassium hydroxide solution [mol / L]
[0275] T: Titration of alcoholic potassium hydroxide solution [ml]
[0276] f: Titration rate of alcoholic potassium hydroxide solution
[0277] W: sample weight [g]
[0278] ○Compulsory deterioration test
[0279] 5 g of the obtained acrylate was added to a 30 ml glass container, and the water concentration contained in the acrylate was adjusted to 1000 to 3000 wtppm by allowing to stand in a cool and dark place in the atmosphere for severa...
Embodiment 1—1
[0306] In Comparative Example 1-1, the addition of acrylic acid was changed from 180g to 150g, the reaction temperature of dehydration esterification was changed from 100 to 119°C to 100 to 117°C, and the reaction time was changed from 9 hours to 5 hours. Minutes, except that, according to the same method as in Comparative Example 1-1, acrylate was produced and purified by the same method.
[0307] About the obtained acrylate, the compound A1 was quantified by the method similar to Comparative Example 1-1, and the acid value and the acid value after the forced deterioration test were measured. These results are shown in Table 1.
Embodiment 1—2
[0309] In Example 1-1, the crude product obtained after the solvent removal step was put into a 500 ml flask, heated and stirred at 80° C., and water was added to adjust the water concentration to 4000 to 5000 ppm. Then, the flask was sealed, and heating and stirring were continued for 72 hours.
[0310] Then, the crude product was cooled to 40° C. or lower, and toluene and a 20 wt % sodium hydroxide aqueous solution were added for neutralization treatment, and water was further added for extraction and washing.
[0311] 0.2 g of MQ was added to the obtained organic layer, and it heated to 60-80 degreeC under reduced pressure, spraying oxygen-containing gas, and toluene was distilled off.
[0312] After filtration under pressure, acrylate was obtained as a product.
[0313] About the obtained acrylate, the compound A1 was quantified by the method similar to Comparative Example 1-1, and the acid value and the acid value after the forced deterioration test were measured. These...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com