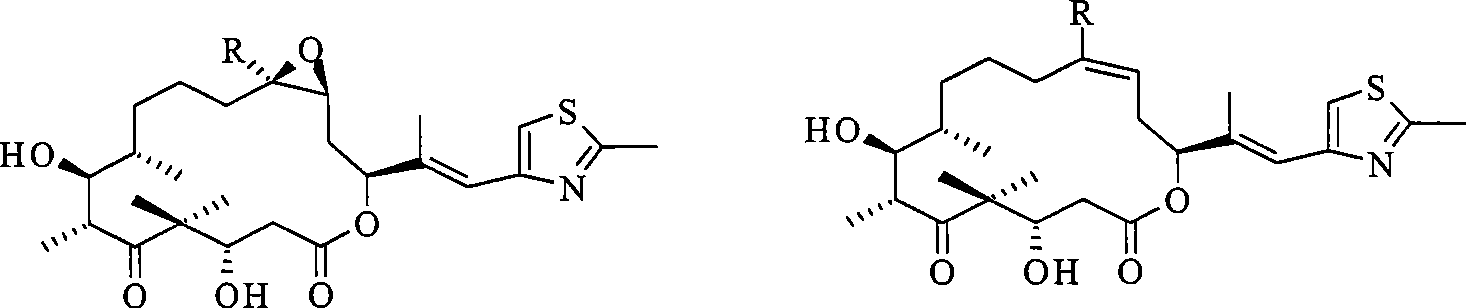
Epothilone freeze-drying composition
A technology of epothilone and its composition, which is applied in the field of epothilone freeze-dried composition and its preparation, can solve the problems of cumbersome and complicated operation, and achieve the effects of good solubility, stable quality and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve 5.0 mg of epothilone B in 500 mg of Solutol HS 15, then add 10 mM NaH, pH 7.0 2 PO4-Na 2 HPO 4 Buffer solution and 500 mg of mannitol were prepared into 5 ml of a clear solution. The solution was sterilized and filtered through a filter membrane with a pore size of 0.2 μm. The filtrate was filled into a vial with a volume of 10 ml under aseptic conditions, and lyophilized Rubber stopper, freeze-dried, after the freeze-drying is completed, aseptically inject sterile dry nitrogen gas to raise the pressure of the freeze-drying chamber to atmospheric pressure, compress the rubber stopper aseptically to provide an airtight aseptic seal, crimp the cap , Labeled, boxed and stored at 2-30°C.
[0036] Just before administration, it is reconstituted with 5ml of water for injection or other aqueous pharmaceutical media, and administered intravenously.
Embodiment 2
[0038] Dissolve 5.0 mg of epothilone B in 500 mg of Solutol HS 15, then add water for injection and 500 mg of mannitol to prepare 5 ml of a clear solution, pass the solution through a filter membrane with a pore size of 0.2 μm, and sterilize and filter the filtrate in a sterile Fill it into a vial with a volume of 10ml under the same conditions, add a freeze-dried rubber stopper under aseptic conditions, and freeze-dry. Aseptically compress the rubber stopper to provide an airtight aseptic seal, crimp the cap, label it, and pack it into a box for storage at 2-30°C.
[0039] Just before administration, reconstitute with 5ml of water for injection or other aqueous pharmaceutical media, and administer intravenously.
Embodiment 3-11
[0041] According to the method of Example 2, the lyophilized composition of the present invention was prepared with the prescription in Table 1.
[0042] Table 1
[0043] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com