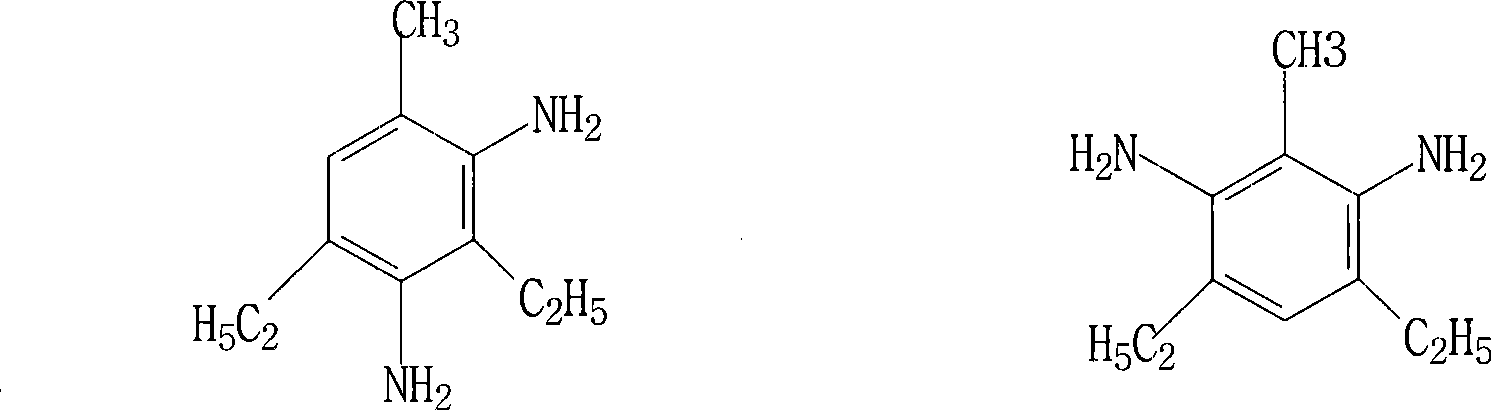Synthesis method of diethyl toluene diamine
A technology of diethyltoluenediamine and a synthesis method, which is used in the preparation of amino compounds from amines, chemical recycling, organic chemistry, etc., can solve problems such as unfavorable human health and environmental protection, expensive alkyl aluminum, and environmental pollution of waste materials. , to achieve the effect of being conducive to environmental protection, reducing catalyst costs, and reducing material viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Put 2000g of TDA, 35g of AL powder (20-40 mesh), 15g of Zn powder (40 mesh), 50g of aluminum trichloride (anhydrous), and 10g of diethylaluminum chloride into a stirred 51 autoclave, and heat together. When the temperature rises to 150°C, hydrogen will be released, and the hydrogen will basically emit light at 200°C for nearly 1 hour; pass through ethylene to purge 2-3mim; continue to heat up to 320°C, fill with pressurized ethylene, and the pressure is 6.5-7.0Mpa , The amount of ethylene is about 990g, and the ethylene is completely absorbed after about half an hour. Then lower the temperature and reduce the pressure, add diphenyl ether to dilute the reactant, filter at 0.2-0.3Mpa, 150°C, and distill the filtrate to obtain the product 3.5-diethyl-2.4(2.6)-diaminotoluene, the yield is 73%, the product The content was analyzed by high pressure liquid chromatography to be 95%.
Embodiment 2
[0035] Put TDA2000g into the reactor, Al powder (20-40 mesh) 40g, Zn powder (40 mesh) 15g, aluminum trichloride
[0036] (anhydrous) 55g, diethylaluminum chloride 15g, all the other are identical with embodiment 1, add phenoxybiphenyl to dilute reactant before filtration, record yield and be 75%, product is analyzed by high pressure liquid chromatography and content is 97%.
Embodiment 3
[0038] Drop into reactor TDA2000g, AL powder (20-40 order) 35g, Zn powder (40 order) 15g, aluminum trichloride (anhydrous) 60g, monochlorodiethylaluminum 18g, all the other are identical with embodiment 1, measure Obtain product yield 80%, content 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com