Antivirus andrographolide derivative and preparation thereof
A technology of andrographolide and derivatives is applied in the field of medicine and achieves the effects of simple preparation process and stable drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0016] Yanhuning rimantadine synthesis process:
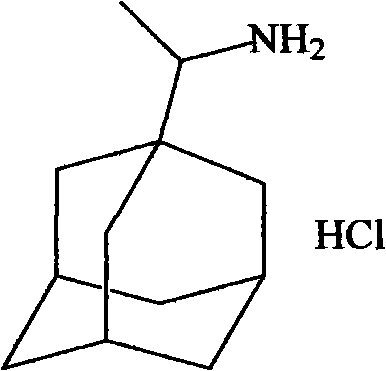
[0017] 1) Riantadine:
[0018] The structural formula is as follows: CAS 1501-84-4
[0019]
[0020] MW 215.7 C 12 h 21 N HCl
[0021] Physical and chemical properties: slightly soluble in water, soluble in chloroform, MP300℃. Hydrochloride was dissolved in 5 times ethanol, 18 times chloroform, 2.5 times water. PH 3.5-5, 1.87g rimantadine hydrochloride (0.01mol) + 5ml distilled water were dissolved, 25% NaOH (1.6ml, theoretical amount) was used to adjust the pH to 3-5, a large amount of solids were precipitated, washed with water, and dried. MP 225~235℃
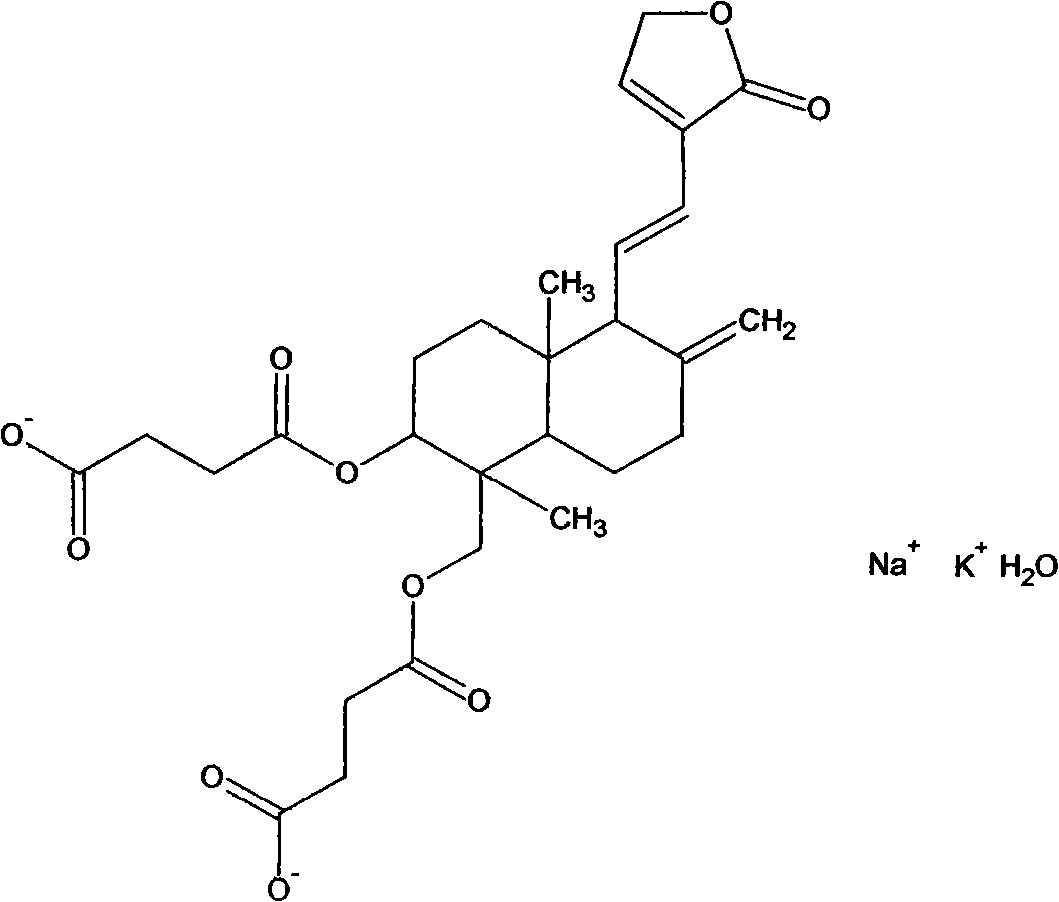
[0022] 2) Yanhuning:
[0023] The structural formula is as follows:
[0024]
[0025] C 28 h 34 KNaO 10 h 2 O MW610.6 PH6.0~8.0 unstable.
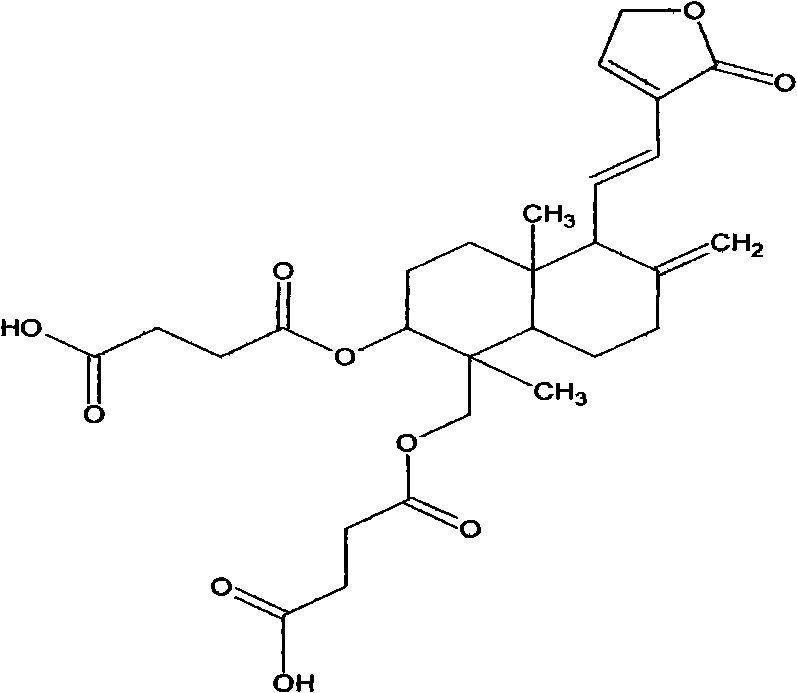
[0026]
[0027] Andrographolide disuccinic acid half ester C28H36O10 MW 532.5 Measured value MP 102~110℃ (literature value 137~140℃).
example 1
[0029] Add 2.67 g (0.005 mol) of andrographolide disuccinic acid half ester into 40 ml of chloroform, and slightly heat (35° C.) to dissolve. Add 1.51 g (0.01 mol) of rimantadine into 30 ml of chloroform and dissolve. Under stirring at room temperature, the rimantadine / chloroform solution was slowly added to the andrographolide disuccinate half ester / chloroform solution. Stirring was continued, and a large number of slightly yellow crystals were precipitated. After standing for 1 hour, it was filtered, dried and pulverized. 3.9 g of light yellow powder was obtained, yield 82%, MP 215-220°C, namely 14-dehydroxy-11,12-didehydroandrographolide-3,19-disuccinic acid half ester rimantadine salt.
example 2
[0031] Add 2.67g (0.005mol) of andrographolide disuccinic acid half ester into 30ml of ethanol, and heat (40°C) to dissolve. Add 1.51 g (0.01 mol) of rimantadine into 20 ml of ethanol to dissolve. Under stirring at room temperature, slowly add the rimantadine / ethanol solution to the andrographolide disuccinate half ester / ethanol solution. Stirring was continued, and a large number of slightly yellow crystals were precipitated. After standing for 1 hour, it was filtered, dried and pulverized. Obtain 4 grams of light yellow powder, yield 83%, HPLC content 98%, MP 215~220 ℃, namely 14-dehydroxyl-11,12-didehydroandrographolide-3,19-disuccinic acid half ester Riantadine salt.
[0032] The salt composed of 14-dehydroxy-11,12-didehydroandrographolide-3,19-disuccinic acid half ester and rimantadine is 14-dehydroxy-11,12-didehydroandrographolide- 3,19-disuccinic acid half ester rimantadine salt, its reaction equation is as follows:
[0033]
[0034] Analysis by proton nuclear m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com