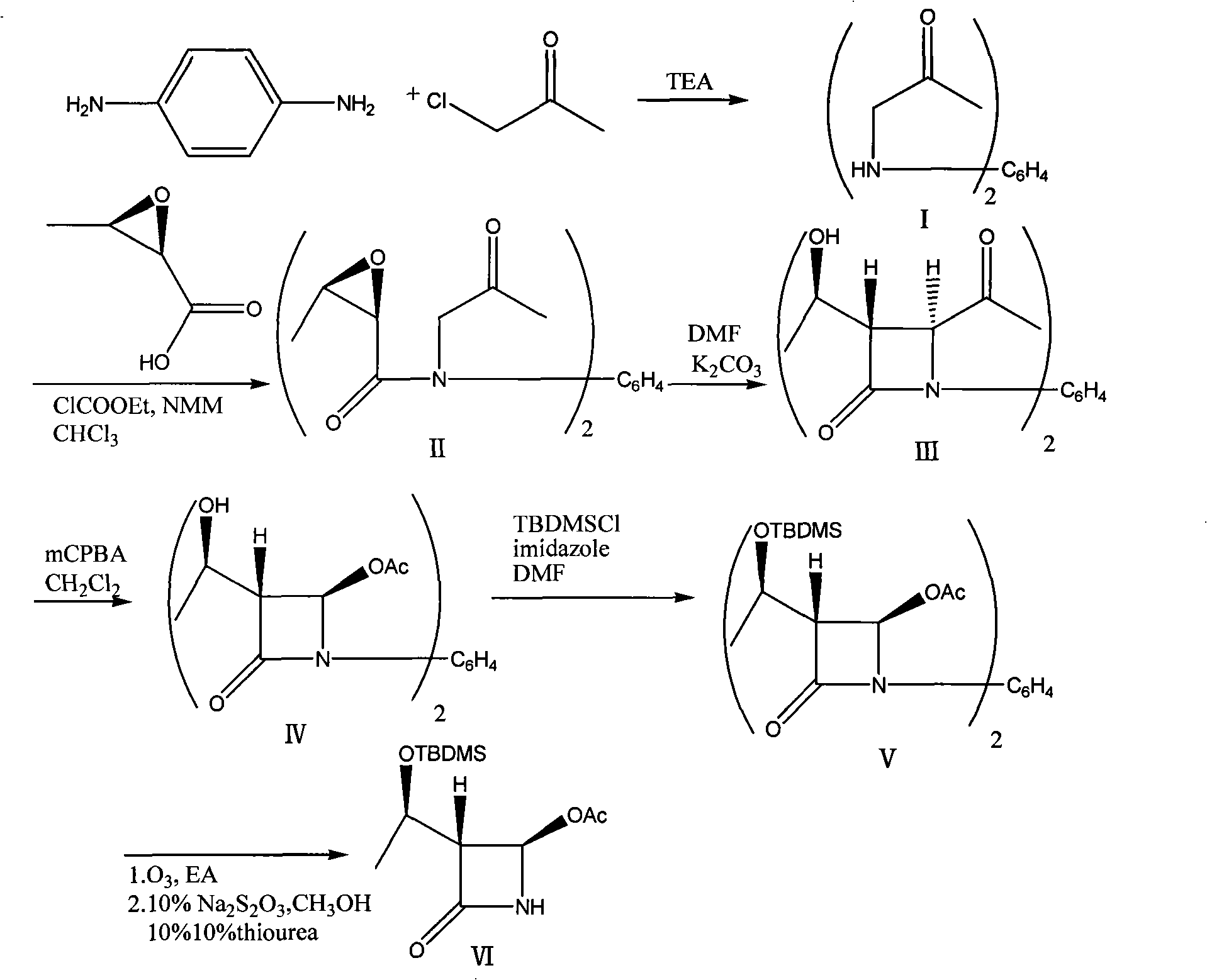
Process for synthesizing 4- acetoxy-2-azetidinone
A technology of azetidinone and acetoxy, which is applied in the field of compound synthesis technology, can solve the problems of environmental hazards and unfavorable industrial production, and achieve the effects of environmental friendliness, mild reaction conditions and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Add 21.6g of p-phenylenediamine and 200ml of triethylamine in a 500ml three-necked flask, add 36.8g of chloroacetone dropwise under reflux, react under reflux for 2 hours, distill off triethylamine, and cool the reactant to 50°C. Add 80ml of water, filter, and dry the filter cake to obtain 40.0g of 1,4-bis[(oxypropyl)amino]benzene with a yield of 90.9%, which can be directly used in the next reaction.
[0024] 2) Add 350ml chloroform and 10.2g epoxybutyric acid into a 1000ml three-necked flask, cool to -10°C, add 10.0g nitrogen methylmorpholine and 10.8g methyl chloroacetate, react for 1 hour, add 1,4-di [(Oxopropyl)amino]benzene 11.0g, heat up to 40°C and react for 1 hour, add 200ml of 1N HCl, separate the layers, wash the chloroform layer with 100ml of saturated sodium bicarbonate and 100ml of saturated brine, and wash with 10g of anhydrous magnesium sulfate Dry, filter, evaporate the filtrate to dryness under reduced pressure, add 300ml of n-hexane, stir to obtain...
Embodiment 2
[0030] 1) Add 21.6g of p-phenylenediamine and 200ml of triethylamine in a 500ml three-necked flask, add 55.2g of chloroacetone dropwise under reflux, and react under reflux for 3 hours. The reactant is cooled to 60°C, and triethylamine is distilled off. Add 80ml of water, filter, and dry the filter cake to obtain 41.1g of 1,4-bis[(oxypropyl)amino]benzene with a yield of 93.5%, which can be directly used in the next reaction.
[0031] 2) Add 350ml chloroform and 10.2g epoxybutyric acid to a 1000ml three-necked flask, cool to -15°C, add 10.0g nitrogen methylmorpholine and 10.8g methyl chloroacetate, react for 2 hours, add 1,4-di [(Oxopropyl) amino] 11.0 g of benzene, heated up to 50 ° C for 2 hours, added 200 ml of 1N HCl, separated, the chloroform layer was washed with 100 ml of saturated sodium bicarbonate and 100 ml of saturated brine, and 10 g of anhydrous magnesium sulfate Dry, filter, evaporate the filtrate to dryness under reduced pressure, add 300ml of n-hexane, stir to ...
Embodiment 3
[0037] 1) Add 21.6g of p-phenylenediamine and 200ml of triethylamine into a 500ml three-necked flask, add 92g of chloroacetone dropwise under reflux, and react under reflux for 4 hours, cool the reactant to 60°C, distill out triethylamine, add 80ml of water was filtered and the filter cake was dried to obtain 42.0g of 1,4-bis[(oxypropyl)amino]benzene with a yield of 95.5%, which could be directly used in the next reaction.
[0038] 2) Add 350ml chloroform and 5.1g epoxybutyric acid into a 1000ml three-necked flask, cool to -20°C, add 10.0g nitrogen methylmorpholine and 10.8g methyl chloroacetate, react for 1 hour, add 1,4-di [(Oxopropyl)amino]benzene 11.0g, heat up to 40°C and react for 1 hour, add 200ml of 1N HCl, separate the layers, wash the chloroform layer with 100ml of saturated sodium bicarbonate and 100ml of saturated brine, and wash with 10g of anhydrous magnesium sulfate Dry, filter, evaporate the filtrate to dryness under reduced pressure, add 300ml of n-hexane, sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com