Method for preparing nano micelle formulation of anthracene nucleus antineoplastic antibiotic
A nano-micelle, anti-tumor technology, applied in anti-tumor drugs, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve the problems of complex process and difficult control of industrial production, and can prolong the circulation time and improve the tumor tissue. Targeted, toxicity-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Encapsulation efficiency of ADM-PEG2000-DSPE (polyethylene glycol derivatized stearoylphosphatidylethanolamine entrapped adriamycin hydrochloride) micelles
[0053] See Table 1 for the prescription:
[0054] Table 1 Example 1: Encapsulation efficiency of ADM-PEG2000-DSPE micelles
[0055]
[0056] Preparation Process:
[0057] Original process: According to the ratio of traditional Chinese medicine in the above prescription, weigh ADM (doxorubicin hydrochloride) and dissolve it in methanol (2mg / ml), and weigh PEG2000-DSPE. (polyethylene glycol derivatized stearoylphosphatidylethanolamine) , dissolved in an appropriate amount of chloroform, placed in a 100ml eggplant-shaped bottle. Set up a rotary evaporator to remove the organic solvent and form a thin and uniform phospholipid film on the surface of the eggplant-shaped bottle. Add the phosphate buffer solution into an eggplant-shaped bottle, shake and hydrate at 30°C for 1 hour, protect with nitrogen, f...
Embodiment 2
[0059] Example 2: In vitro release test of ADM-PEG2000-DSPE micelles.
[0060] The release rate of ADM from PEG2000-DSPE micelles was determined by dissolution method and detected by HPLC.
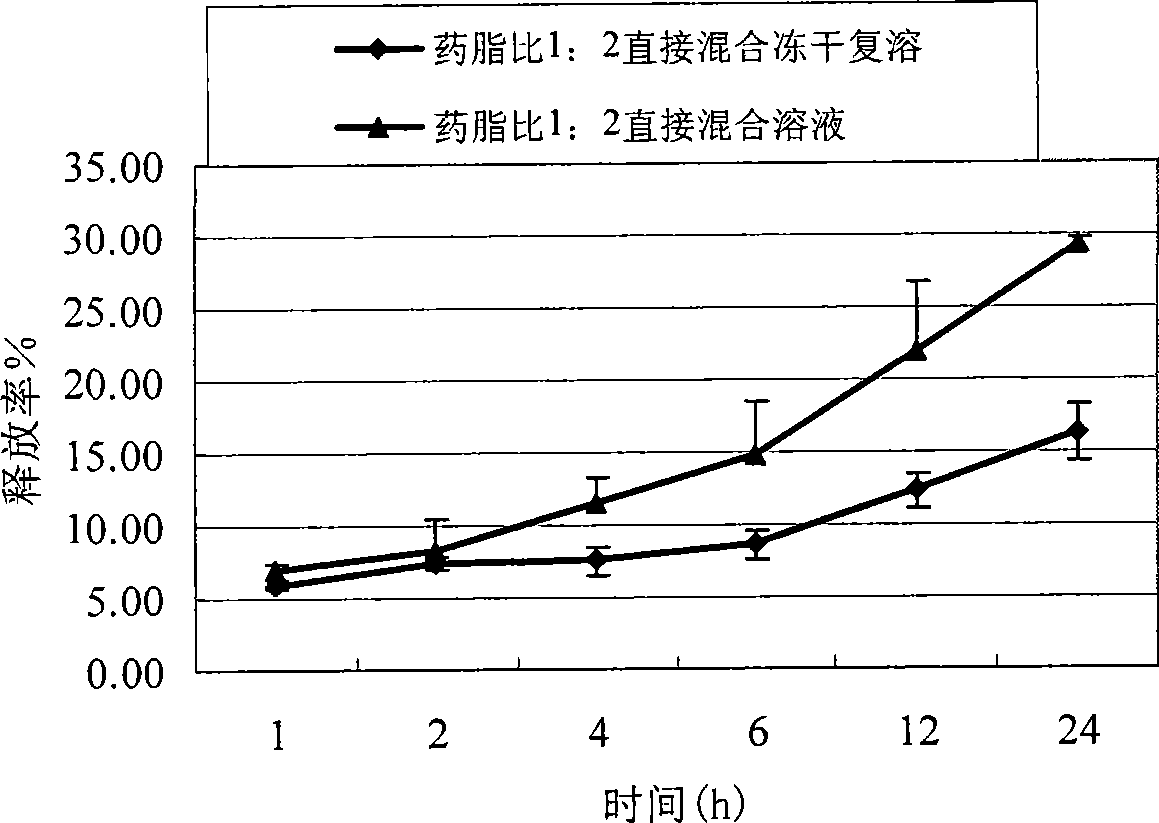
[0061] Place the prepared ADM-PEG2000-DSPE micelles 1ml (1mg / ml) in the dialysis bag (molecular weight cut-off 1,2000-1,4000), place the fastened dialysis bag in the rotating basket of the drug dissolution apparatus, The external fluid for dialysis was 150ml of PBS buffer, and three samples were prepared in parallel. Run at 37°C and 50 rpm for 24 hours. Samples were taken at 1h, 2h, 4h, 6h, 12h, and 24h. Fresh PBS0.2ml. Samples taken at fixed points were entered into HPLC, and compared with the standard curve, the release percentage was obtained. see results figure 1 . Among them, when the ratio of lipid to drug is 1:1, the release rate in 24 hours is 29%; when the ratio of lipid to drug is 2:1, the release rate in 24 hours is 16%; there is no burst release phenomenon.
Embodiment 3
[0062] Example 3: In vitro cytotoxicity test of doxorubicin hydrochloride micellar preparation.
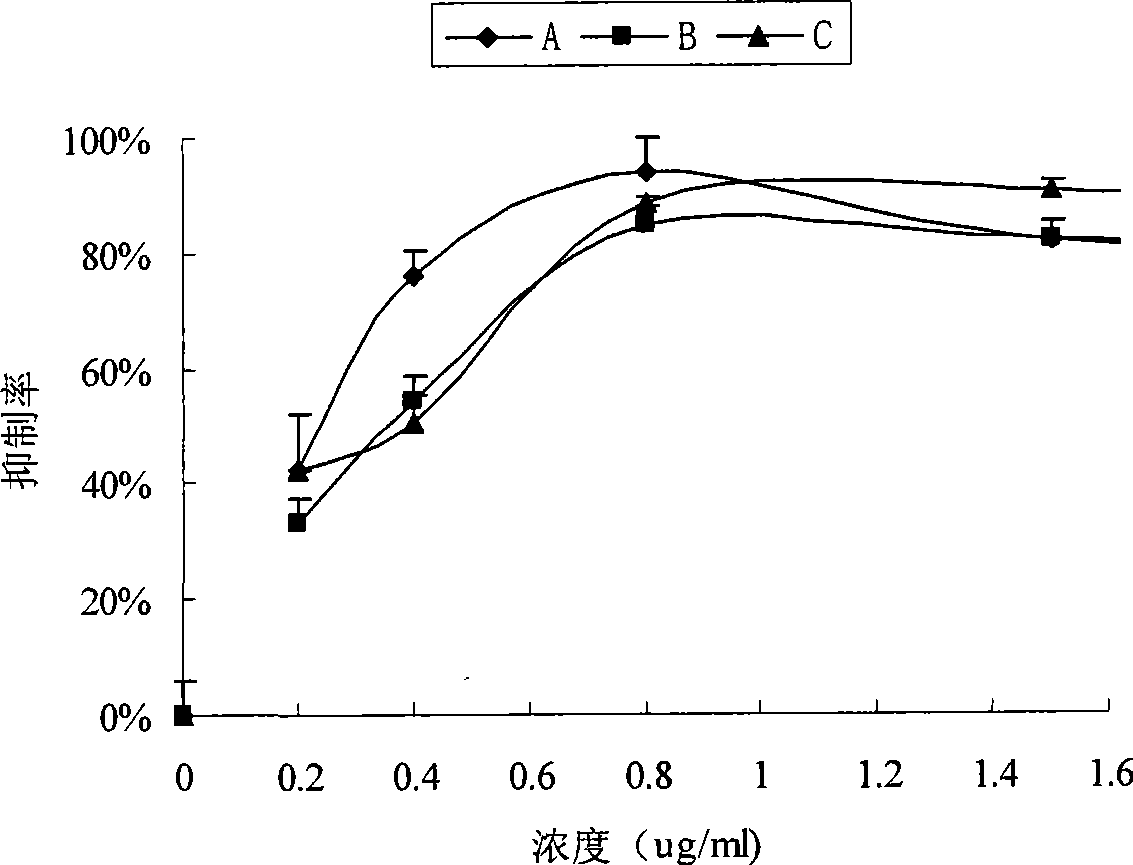
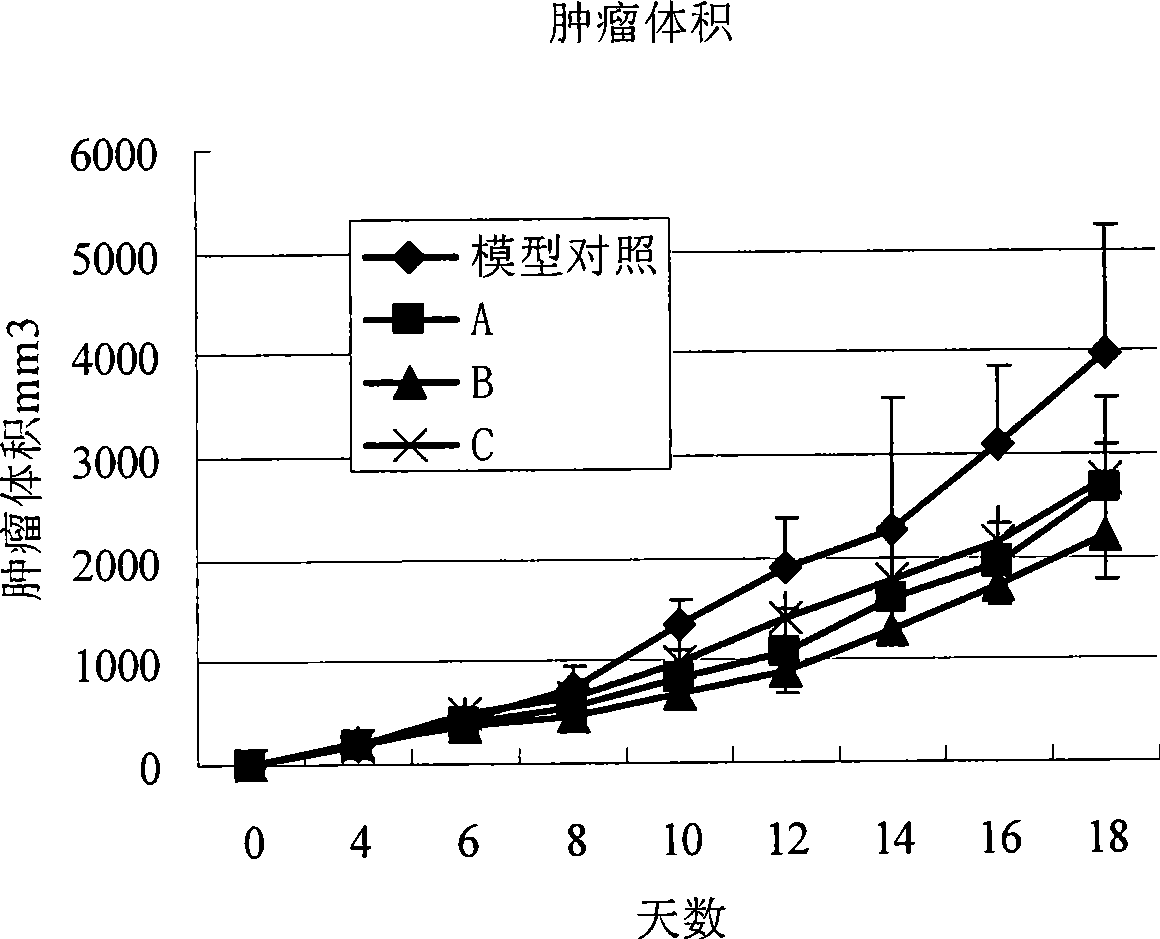
[0063] The antitumor effect of the nano-micelle preparation of anthracycline antitumor antibiotic prepared by the present invention is tested by in vitro cytotoxicity test and in vivo tumor growth inhibition test.
[0064] A549 cells (ATCC) according to 8.0*10 3 Inoculate each well into a 96-well plate, culture overnight, wash off the medium, and add 5ul of the following samples with different doxorubicin concentrations: free doxorubicin (C sample), new technology ADM-PEG2000-DSPE solution (A sample ), the new process ADM-PEG2000-DSPE freeze-dried reconstituted sample (B sample), each sample has three replicate holes. Add 100ul culture medium containing 10% fetal calf serum to each well, incubate at 37°C, 5% CO 2 Continue culturing for 48 h in the incubator. Take out the cells at each set time point, add MTT20ul (5mg / ml) to each well, and after culturing for 4 hours, add 150ul ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com