Salvianolic acid controlled porosity osmotic pump tablets and method of preparing the same
A technology of osmotic pump controlled release and salvianolic acid, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery. It can solve the problems of fast distribution, difficulty in maintaining effective blood drug concentration, and poor compliance. The effect of low requirements, prolonging the effective time of action, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
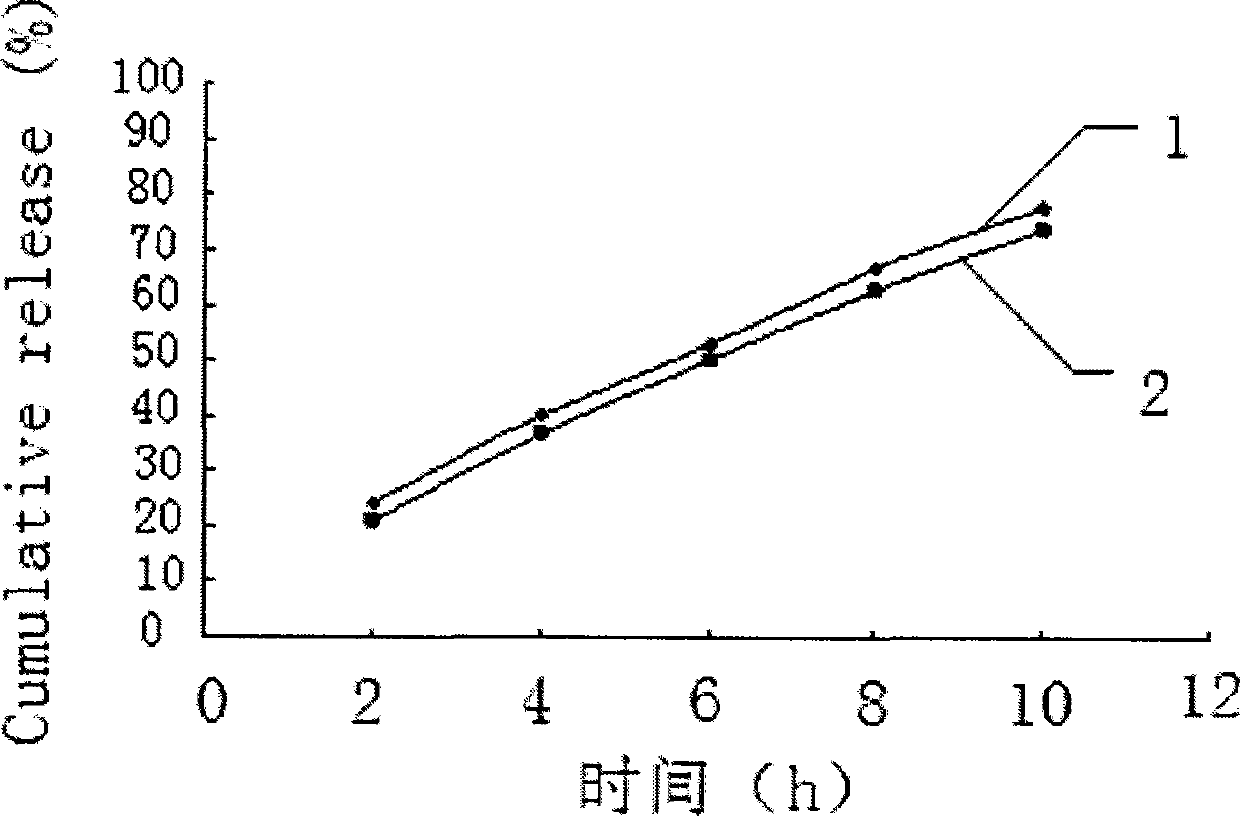
Embodiment 1
[0028] Embodiment 1 prepares tablet core materials according to the following formula:
[0029] Salvianolic acid 50 75 100mg
[0030] Sucrose 75 112.5 150mg
[0031] Lactose 75 112.5 150mg
[0032] Medicinal alcohol (95% ethanol) appropriate amount
[0033] Magnesium stearate 1.0 1.5 2mg
[0034] Wherein, the consumption of medicinal alcohol refers to pharmaceutical routines, as long as the purpose of making the tablet core material into a soft material can be achieved. All reagents are commercially available reagents commonly used in this field, and the following examples are the same.
[0035] Pass the materials for the tablet core through a 60-mesh sieve, mix evenly according to the proportion, add 95% ethanol soft material, pass through a 20-mesh sieve to granulate, dry at 40°C for 5 minutes, pass through a 20-mesh sieve for granulation, add lubricant and mix well, and use Press a 9.5mm punch to make a tablet core with a hardness of 6-7kg.
[0036] Prepare the coatin...
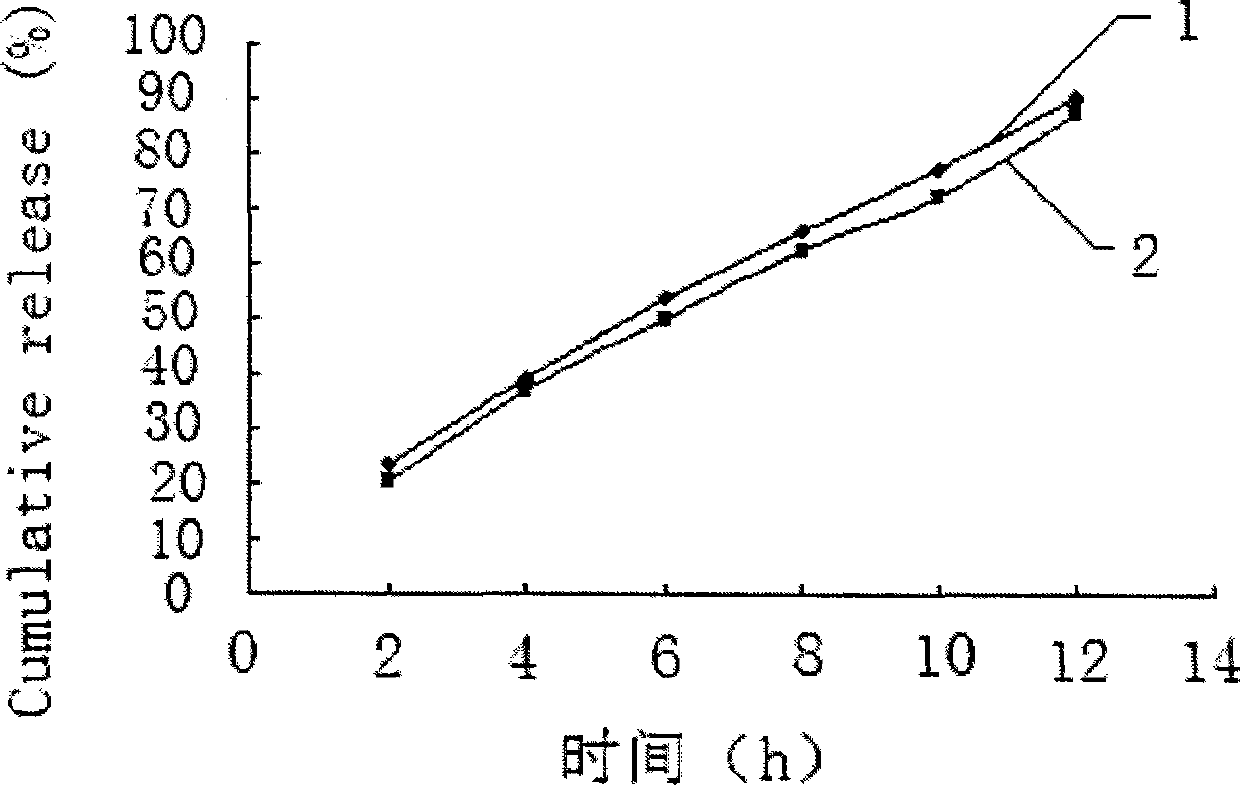
Embodiment 2
[0045] Embodiment 2 prepares tablet core materials according to the following formula:
[0046] Salvianolic acid 50 75 100mg
[0047] Lactose 75 112.5 150mg
[0048] Sodium chloride 75 112.5 150mg
[0049] Medicinal alcohol (95% ethanol) appropriate amount
[0050] Magnesium stearate 1.0 1.5 2mg
[0051] Pass the materials for the tablet core through a 60-mesh sieve, mix evenly according to the proportion, add 95% ethanol soft material, pass through a 20-mesh sieve to granulate, dry at 40°C for 5 minutes, pass through a 20-mesh sieve for granulation, add lubricant and mix well, and use Press a 9.5mm punch to make a tablet core with a hardness of 6-7kg.
[0052] Prepare the coating solution according to the following formula:
[0053] Cellulose acetate 30mg
[0054]Polyethylene glycol 400 6ml
[0055] Diethyl phthalate 9ml
[0056] Acetone 800ml
[0057] Isopropanol 200ml
[0058] Weigh 30 mg of cellulose acetate, measure 6 ml of polyethylene glycol 400, and 9 ml of d...
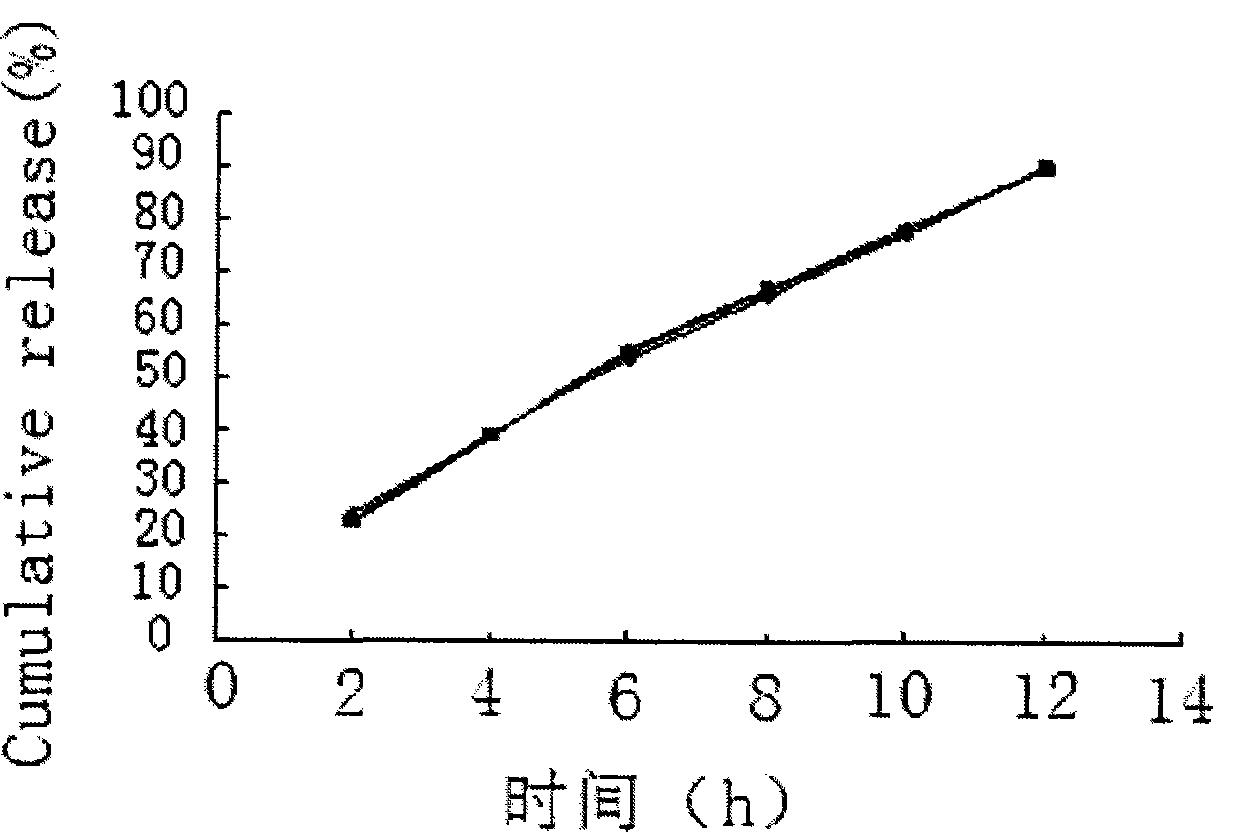
Embodiment 3
[0059] Embodiment 3 prepares tablet core material according to following formula:
[0060] Salvianolic acid 50 75 100mg
[0061] Mannitol 75 112.5 150mg
[0062] Sodium chloride 75 112.5 150mg
[0063] Appropriate amount of medicinal alcohol
[0064] Magnesium stearate 1.0 1.5 2mg
[0065] Pass the materials for the tablet core through a 60-mesh sieve, mix evenly according to the proportion, add soft materials made of anhydrous ethanol, pass through a 20-mesh sieve to granulate, dry at 40°C for 5 minutes, pass through a 20-mesh sieve for granulation, add lubricant and mix well, use Press a 9.5mm punch to make a tablet core with a hardness of 6-7kg / mm 2 .
[0066] Prepare the coating solution according to the following formula:
[0067] Cellulose acetate 30mg
[0068] Macrogol 400 9ml
[0069] Diethyl phthalate 3ml
[0070] Acetone 800ml
[0071] Isopropanol 200ml
[0072] Weigh 30 mg of cellulose acetate, 9 ml of polyethylene glycol 400, and 3 ml of diethyl phthalat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com