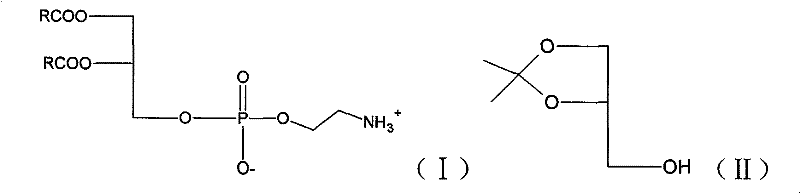
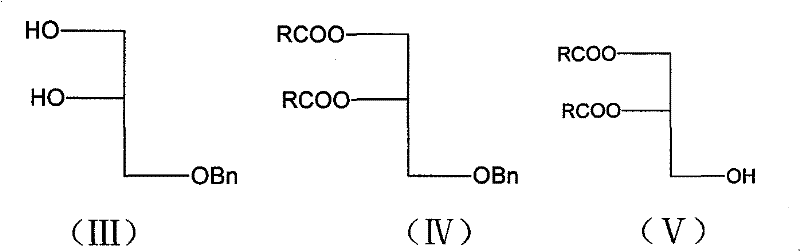
Method for preparing bi-axungia acyl-phosphatidylethanolamine
A technology of stearyl phosphatidylethanolamine and benzyl distearoylglyceride, which is applied in the direction of phosphorus organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 2: Preparation of isopropylidene-D-mannitol
[0043] 100g (0.73mol) of zinc chloride was dissolved in 500ml of acetone solution, heated to reflux for 30 minutes, added 50g (0.27mol) of D-mannitol to continue the reaction for 2 hours, then added 200ml of chloroform, and extracted with 200ml of saturated sodium chloride for phase separation. Add 400 ml of 5% ammonia solution to the organic phase, dry the organic phase with anhydrous magnesium sulfate, and concentrate by rotary evaporation to obtain 49 g of white solid product isopropylidene-D-mannitol.
Embodiment 2
[0044] Embodiment 3: preparation isopropylidene glycerol
[0045] Dissolve 53.9g (0.25mol) of sodium periodate in 430ml of water, add 36g (0.14mol) of isopropylidene D-mannitol in 180ml of isopropanol solution, control the temperature at 35°C, react for 50 minutes, and then add 860ml isopropanol, filter the insoluble matter, add 15.8 g (0.42 mol) of sodium borohydride to the filtrate, continue the reaction for 30 minutes, and control the temperature at 40 ° C, then extract, dry, and concentrate under reduced pressure to obtain a yellow-green liquid, which is then reduced to Pressure distillation obtained 32 g of isopropylidene glycerol. Bp. 10mm 77-79°C, [α] 20 D +15.2.
Embodiment 3
[0046] Embodiment 4: preparation isopropylidene glycerol
[0047] Dissolve 44.9g (0.21mol) of sodium periodate in 225ml of water, add 36g (0.14mol) of isopropylidene-D-mannitol in 150ml of methanol solution, control the temperature at 40°C, react for 20 minutes, then add 113ml of methanol , filter the insoluble matter, add 11.7g (0.31mol) of sodium borohydride to the filtrate, continue the reaction for 10 minutes, control the temperature at 60°C, then extract, dry, and concentrate under reduced pressure to obtain a yellow-green liquid, which is then obtained by distillation under reduced pressure 30.1 g of isopropylidene glycerol. Bp. 10mm 77-79°C, [α] 20 D +15.21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com