Oxymatrine nano preparations for liver cell targeting injection and preparation thereof
A technology of nano-preparation and matrine, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, antiviral agents, etc., can solve the problems of increased adverse drug reactions, decreased therapeutic effect, and high dosage problem, achieve the effect of increasing residence time, improving curative effect and slowing release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A. Matrine 1g
[0028] B. Lecithin 3.6g
[0029] C. Stearic acid 0.4g
[0030] D. Mannitol 4g
[0031] Stirring speed: 1000rpm
[0032] Stirring temperature: 60°C
[0033] Dropping speed: 0.1ml / min
[0034] Dispersion liquid temperature: 0~4℃
[0035] Weigh matrine, lecithin, stearic acid, add 10ml of ethanol, heat to dissolve, slowly drop into heated water with stirring, stir to evaporate the solvent, form a colloidal solution, disperse with ice water to form a SLN suspension, 0.22 Filter through a microporous membrane, add mannitol to mix, dilute to 100ml, sonicate, freeze-dry and store.
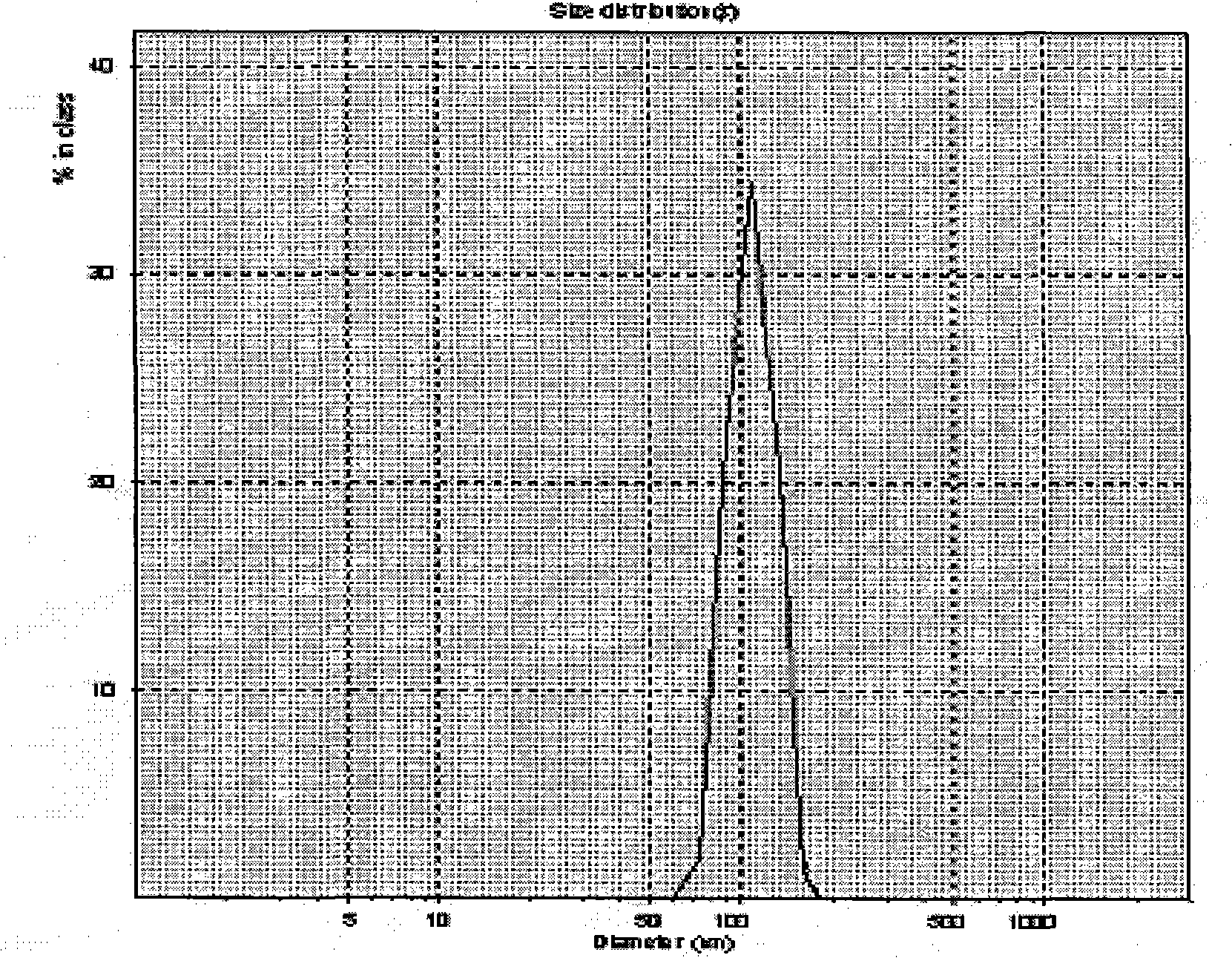
[0036] After adding water to the obtained matrine nano freeze-dried powder, the particle size distribution range is 104±34nm, the surface potential is -32.6mV, and the encapsulation rate is 81%. The in vitro release study was carried out by dialysis, and the release time was as long as 12 hours, and about 80% was released in 8 hours.
[0037] After administration of 100 mg / k...
Embodiment 2
[0045] Embodiment 2 (No. 2-18)
[0046] Preparation method of matrine nano-preparation for liver cell targeting injection (see Table 3).
[0047] Table 3 Preparation method of matrine nano-preparation for liver cell targeting injection
[0048]
[0049] Specific steps are as follows:
[0050] Weigh matrine, lecithin, stearic acid, add 10ml of ethanol, heat to dissolve, slowly drop into heated water with stirring, stir to evaporate the solvent, form a colloidal solution, disperse with ice water to form a SLN suspension, 0.22 Filter through a microporous membrane, add mannitol to mix, dilute to 100ml, sonicate, freeze-dry and store.
[0051] The particle size of the prepared matrine nano-preparation for liver cell targeting injection (see Table 4).
[0052] The particle size of matrine nano-preparation for liver cell targeting injection prepared in table 4
[0053]
Embodiment 3
[0054] Embodiment 3 (No. 19-25)
[0055] Preparation method of matrine nano-preparation for liver cell targeting injection (see Table 5).
[0056]Table 5 Preparation method of matrine nano-preparation for liver cell targeting injection
[0057]
[0058] Weigh A, B, C, add 10ml of ethanol, heat to dissolve, slowly drop into the heated water containing E under stirring, stir and evaporate the solvent to form a colloidal solution, disperse with ice water to form a SLN suspension, 0.22μ micro Filter through a pore filter, add D to mix, dilute to 100ml, sonicate, freeze-dry and store.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com