Double phosphonic acid salt medicaments and ion chromatographic fractionation analysis method of impurity anion thereof
A technology for separation and analysis of bisphosphonates, which is applied in the field of analysis of bisphosphonates, can solve problems such as cumbersome operations, and achieve the effects of expanding the scope of application and simplifying the process flow.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This example analyzes alendronate sodium and five common anions in alendronate sodium tablets
[0036] Instruments used: Dionex ICS 2000 ion chromatograph (Dionex, USA); EG50 eluent generator; DS6 conductivity detector; Chromeleon 6.5 chromatography workstation; IonPac AG11 guard column (50mm×2mm); IonPac AS11 separation column (250mm×2mm) 2mm); 25μL injection volume.
[0037] Suppressor: Dionex AAES self-regenerating suppressor;
[0038] Eluent: KOH solution, using gradient elution. At 0-2 minutes, the concentration of potassium hydroxide is 1mmol / L; at 2-12 minutes, the concentration is 1mmol / L-18mmol / L; at 12.01-17.00min, the concentration of potassium hydroxide is 1mmol / L.
[0039] Analysis steps:
[0040] ① Baseline surveying and mapping
[0041] The eluent is pumped into the chromatographic separation column to achieve equilibrium, and the eluent output from the chromatographic separation column enters the suppressor, and when it flows into the conductivity fl...
Embodiment 2
[0047] This embodiment is to analyze the etidronate sodium content in the rabbit plasma.
[0048] Apparatus used: same as Example 1.
[0049] Suppressor: same as embodiment 1.
[0050] Eluent: KOH solution. The gradient elution scheme is: when 0-2min, the concentration of 3mmol / L potassium hydroxide is selected; for 2-12min, the concentration is 1mmol / L-30mmol / L potassium hydroxide; L of potassium hydroxide.
[0051] The analysis steps are the same as in Example 1. The plotted spectrum is shown in Figure 2.
[0052] The analysis result is: the content of etidronate sodium in the plasma sample collected 1 hour after the rabbit took the medicine is: 5.0 μg / ml
Embodiment 3
[0054] This embodiment is to analyze the content of risedronate sodium in physiological saline.
[0055] Apparatus used: same as Example 1.
[0056] Suppressor: Dionex ASRS-ULTRA self-regenerating suppressor.
[0057] Eluent: KOH solution. The gradient elution scheme is: at 0-3 minutes, the concentration of potassium hydroxide is 20mmol / L; at 3.01-8.00min, the concentration is 45mmol / L; at 8.01-15.00min, the concentration of potassium hydroxide is 20mmol / L.
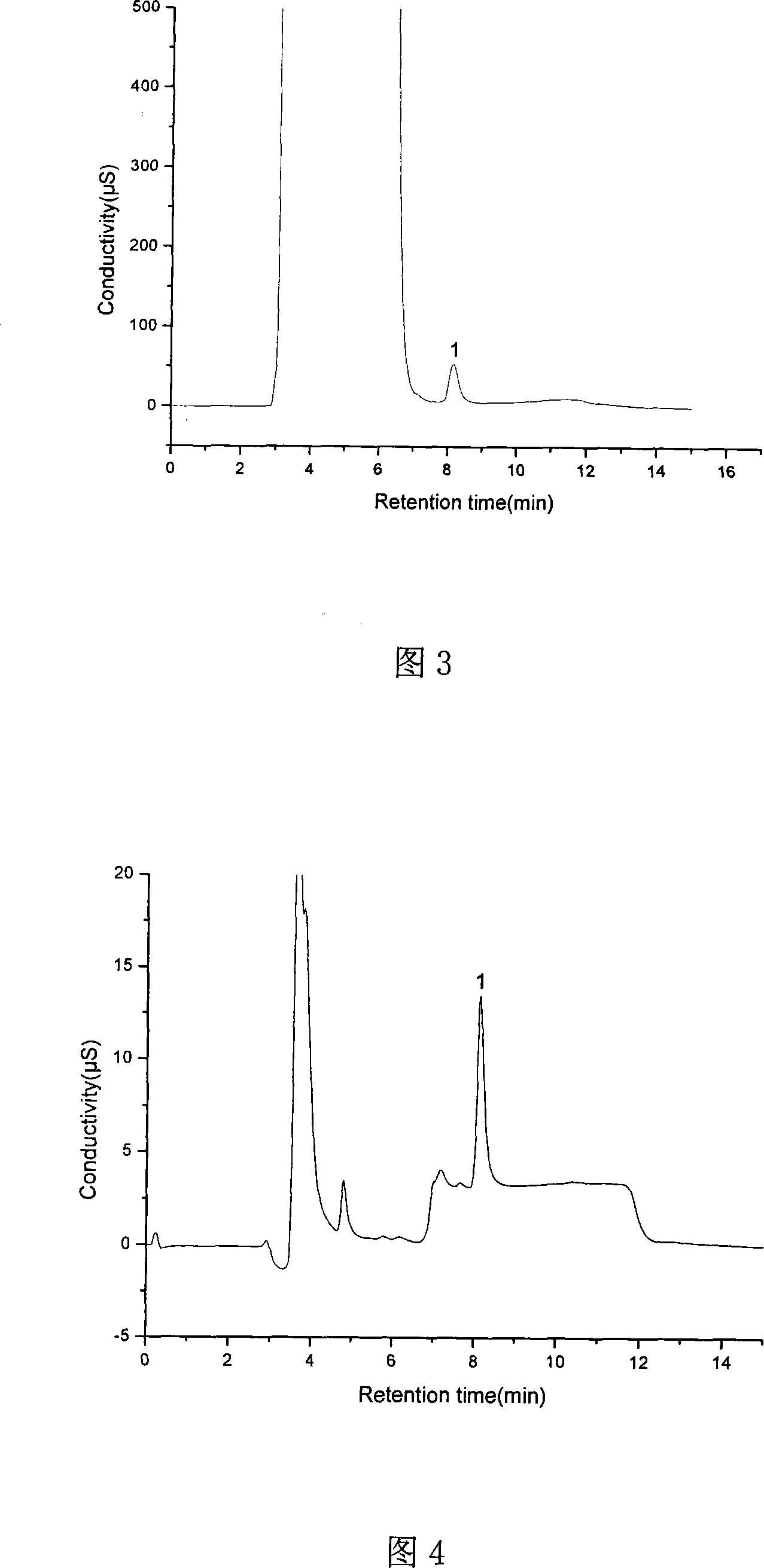
[0058] The analysis steps are the same as in Example 1. The plotted spectrum is shown in Figure 3.
[0059] The analysis results are as follows: the normal saline matrix does not interfere with the determination of risedronate sodium, and the peak shape of the sample chromatographic peak is very good; the average recovery rate of risedronate sodium is 106.4%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com