Biological compatibility magnetic nano crystal for high dissolving and stable distribution in physiologicalbuffer liquid and its making method
A biocompatible, physiological buffer technology, applied in the field of biocompatible magnetic nanocrystals, can solve the problems of solubility and stable dispersion that need to be further improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
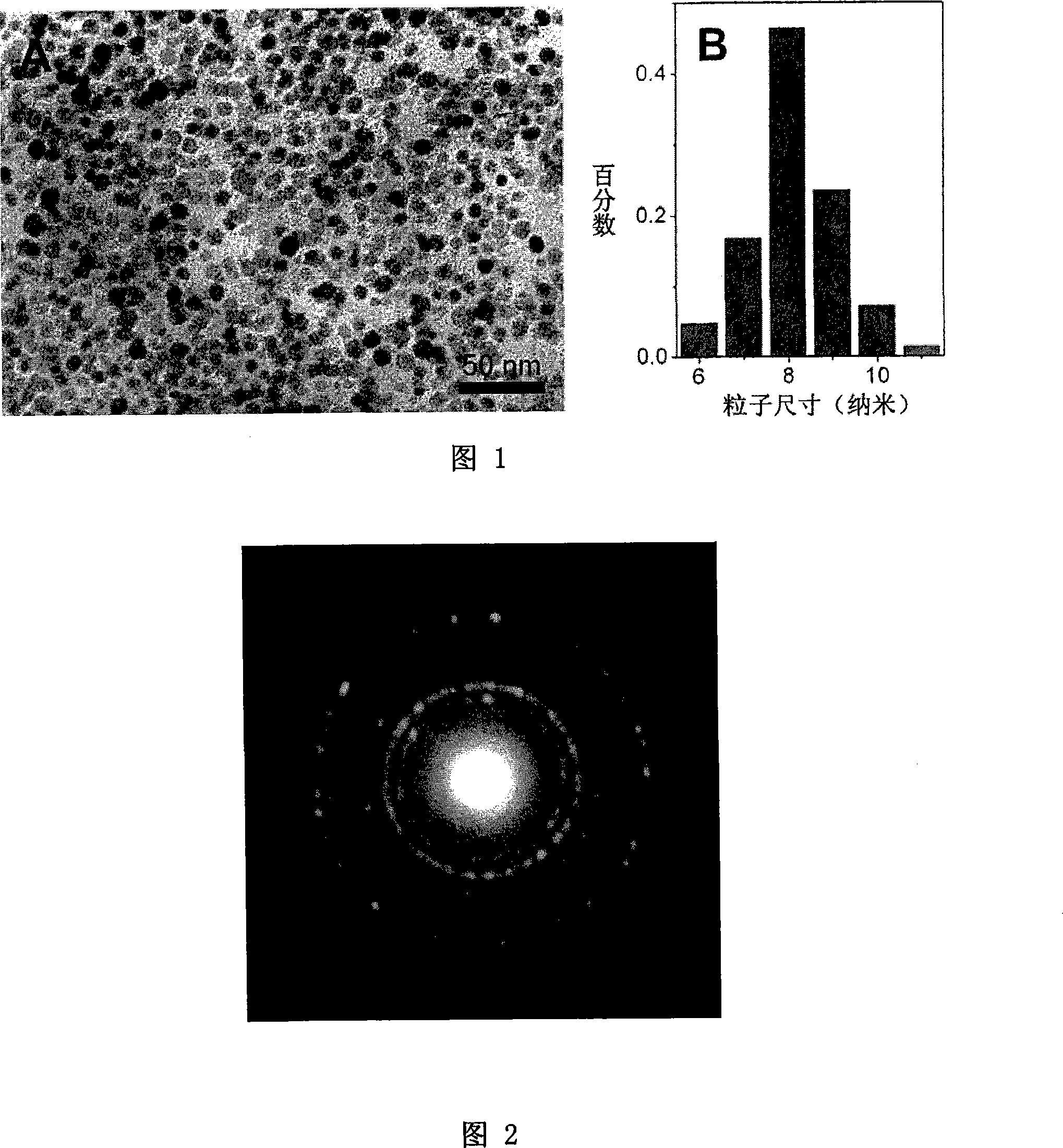
[0061] 1.06g iron acetylacetonate, 12g dicarboxy PEG2000 (prepared according to the document Adv.Mater., 2005, 17 (8), 1001 method), 3.87mL oleylamine were dissolved in 50mL phenyl ether, and then the above solution was transferred to 100mL In the three-necked flask, nitrogen was introduced to remove oxygen for 30 minutes, and the reaction solution was refluxed for 20 hours. The reaction system was cooled to room temperature, and the biocompatible magnetic nanocrystals with carboxyl groups on the surface were precipitated with ether, washed three times, and centrifuged to obtain the biological phase. Capacitive Fe 3 o 4 magnetic nanocrystals. The obtained nanoparticles were dissolved in deionized water, dialyzed for 24 hours, and the obtained solution was precipitated and washed with a mixture of ether and acetone (volume ratio of 3:1), and dried in vacuum to obtain a dry powder that was easy to store and transport. Dissolve the dry powder in deionized water, and use transmi...
Embodiment 2
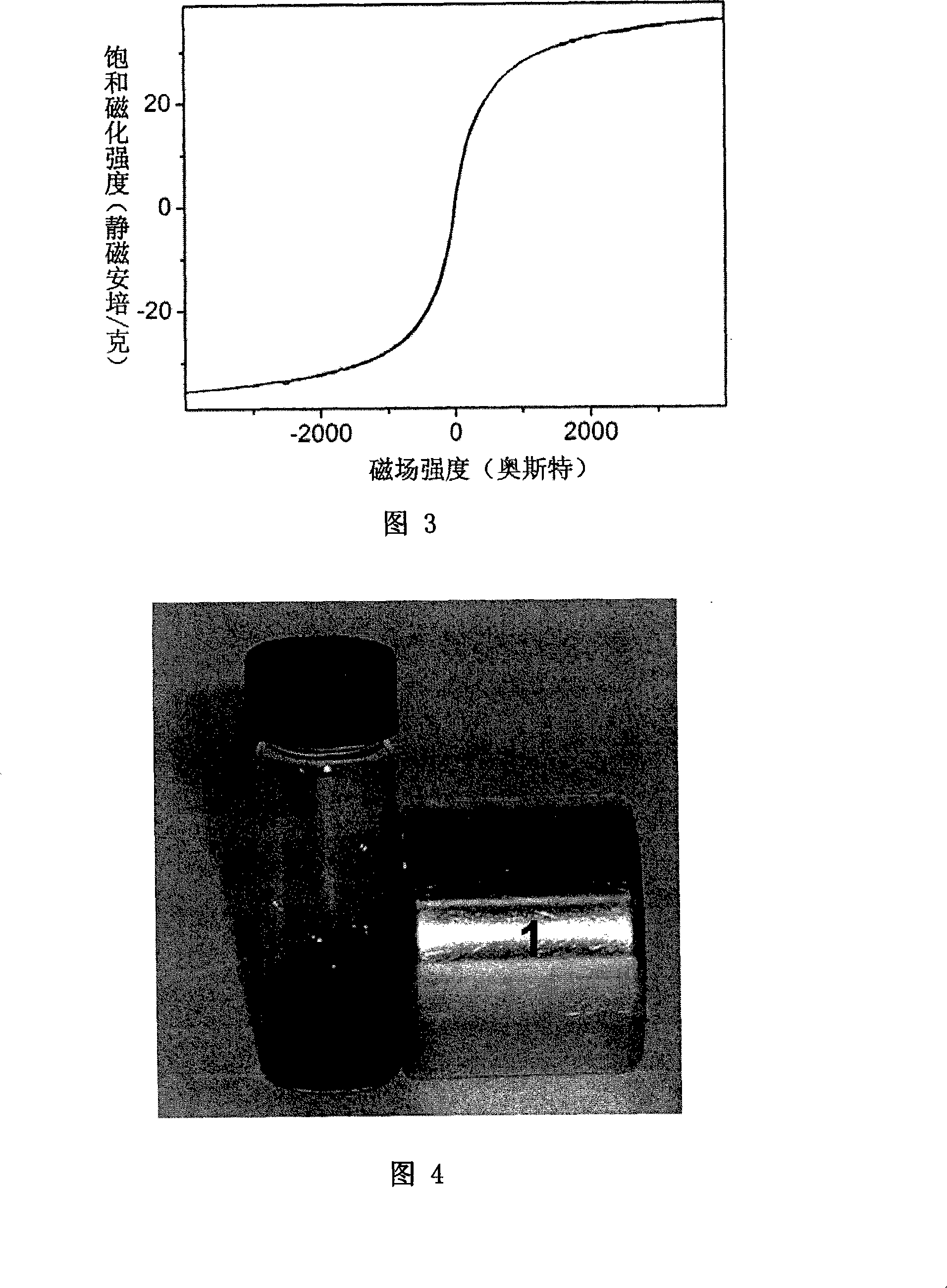
[0063] Place the dry powder sample obtained in Example 1 at room temperature for half a year, weigh 0.6g, add 100mL of 0.01M PBS (phosphate buffer saline, pH=7.4) to dissolve it completely, and prepare a 6g / L magnetic fluid. After the fluid was placed for half a year, there was no precipitation. Accompanying drawing 4 is the photo that this ferrofluid is placed next to the magnet after half a year.
Embodiment 3
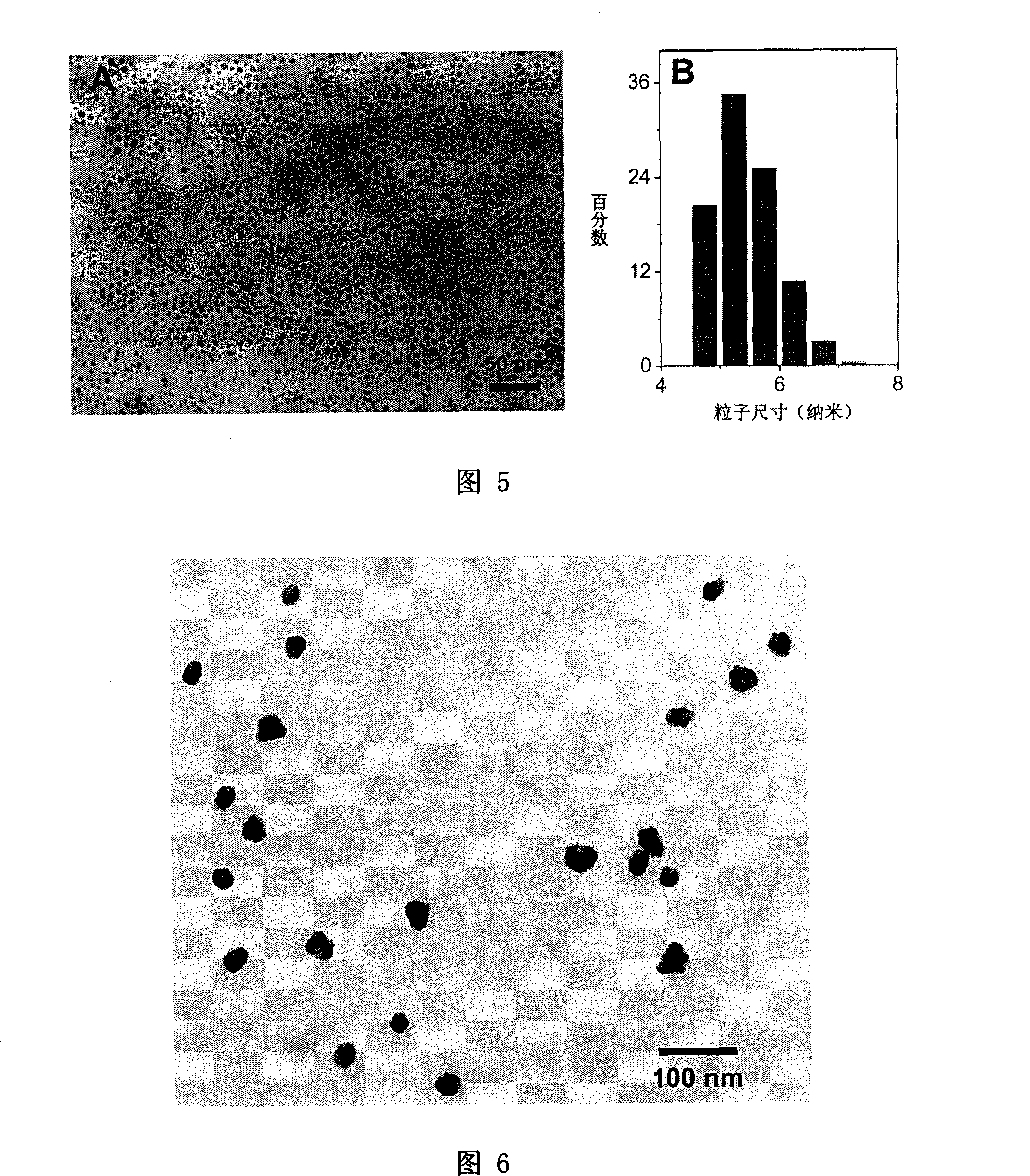
[0065] 0.53g iron acetylacetonate, 6g monocarboxy PEG2000 (prepared according to the document Adv.Mater., 2005, 17 (8), 1001 method) and 1.93mL oleylamine were dissolved in 25mL phenyl ether to make a reaction solution, and then the reaction solution Transfer to a 50mL three-neck flask, fill with nitrogen to remove oxygen for 30 minutes, reflux the reaction solution for 12 hours, cool the reaction system to room temperature, and precipitate the obtained magnetic nanocrystals with ether, wash them three times with ether, and centrifuge to obtain Fe 3 o 4 nanocrystals. After it was naturally dried, it was dissolved in deionized water, and then dialyzed for 24 hours. The dry powder of magnetic nanocrystals obtained by the same method as in Example 1 still shows very good solubility in physiological buffer solution after long-term storage, and the solubility is as high as 50 g / L. Figure 5A is a transmission electron micrograph of the obtained magnetic nanocrystals. Figure 5B is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com