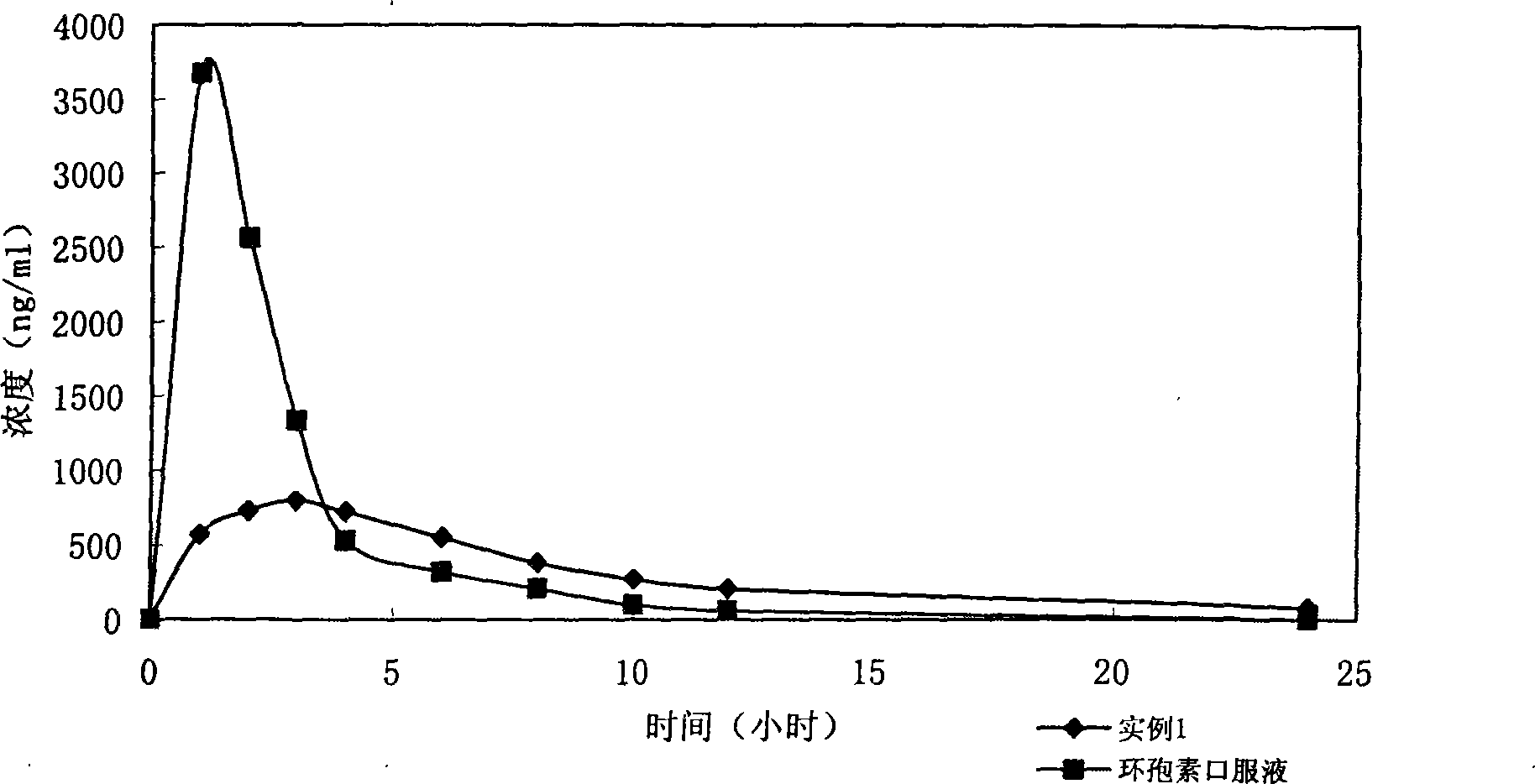
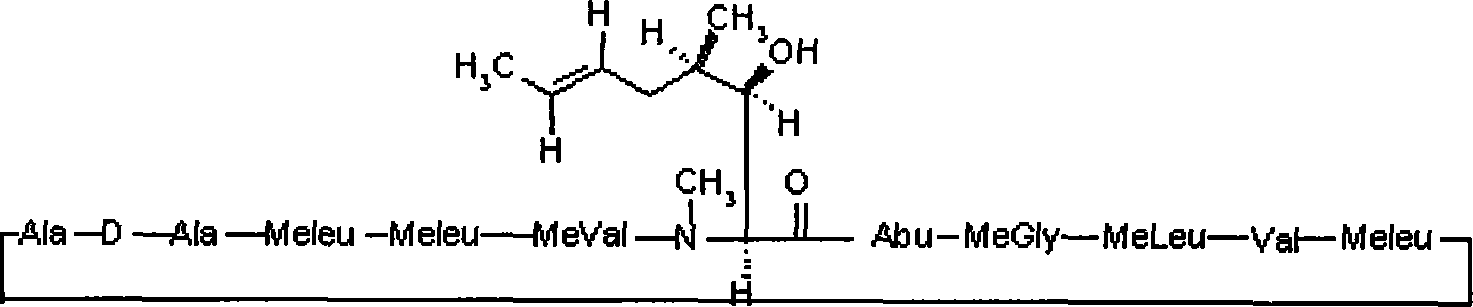
Stage releasing CsA solid tiny milk agent and tiny milk curing method thereof
A technology of cyclosporine and microemulsion, which is applied in the direction of cyclic peptide components, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of complex production, rejection reaction, large loss, etc., and achieve good curing effect and stable blood drug concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0057] Enteric-coated granules
[0058] Cyclosporin A 1kg
[0059] Glyceryl Caprylate 2kg
[0060] Polyoxyethylene hydrogenated castor oil 5kg
[0061] Propylene glycol 3kg
[0062] Polyvinylpyrrolidone K30 4kg
[0063] Micronized silica gel 5kg
[0064] Domestic No. II resin 6kg
[0065] Gastric Dissolved Granules
[0066] Cyclosporin A 1kg
[0067] Glyceryl Caprylate 2kg
[0068] Polyoxyethylene hydrogenated castor oil 5kg
[0069] Propylene glycol 3kg
[0070] Polyvinylpyrrolidone K30 4kg
[0071] Micronized silica gel 5kg
[0072] Domestic IV resin 4kg
[0073] Add cyclosporine A to glyceryl caprylate and propylene glycol, stir to dissolve, add polyoxyethylene hydrogenated castor oil, stir and mix well, add polyvinylpyrrolidone K30 and stir rapidly to make it fully dispersed and dissolved into the solution, Then add micropowder silica gel and stir to make it adsorb into a solid. Dissolve No. II resin with an appropriate amount of ethanol and add it to the mic...
example 2
[0075] Enteric-coated granules
[0076] Cyclosporin A 1kg
[0077] Glyceryl Caprylate 2kg
[0078] Polyoxyethylene hydrogenated castor oil 5kg
[0079] Propylene glycol 3kg
[0080] Polyvinylpyrrolidone K30 4kg
[0081] Micronized silica gel 5kg
[0082] Starch 5kg
[0083] Domestic No. II resin 6kg
[0084] PEG4000.5kg
[0086] Ethanol 80kg
[0087] Gastric Dissolved Granules
[0088] Cyclosporin A 1kg
[0089] Glyceryl Caprylate 2kg
[0090] Polyoxyethylene hydrogenated castor oil 5kg
[0091] Propylene glycol 3kg
[0092] Polyvinylpyrrolidone K30 4kg
[0093] Micronized silica gel 5kg
[0094] Domestic IV resin 4kg
[0095] Add cyclosporine A to glyceryl caprylate and propylene glycol, stir to dissolve, add polyoxyethylene hydrogenated castor oil, stir and mix well, add polyvinylpyrrolidone K30 and stir rapidly to make it fully dispersed and dissolved into the solution, Then add micropowder silica gel and stir to make it adsorb into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com