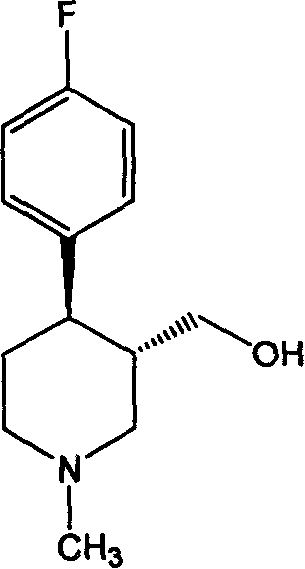
(3S,4R)-4-(fluorophenyl)-3-hydroxymethyl-1-methyl piperidine and split method of antipodism thereof
A technology of methyl piperidine and p-fluorophenyl, which is applied in the field of high-performance liquid phase analysis and separation, can solve the problems of unsatisfactory resolution and unobtainable resolution methods, and achieve the effect of improving symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
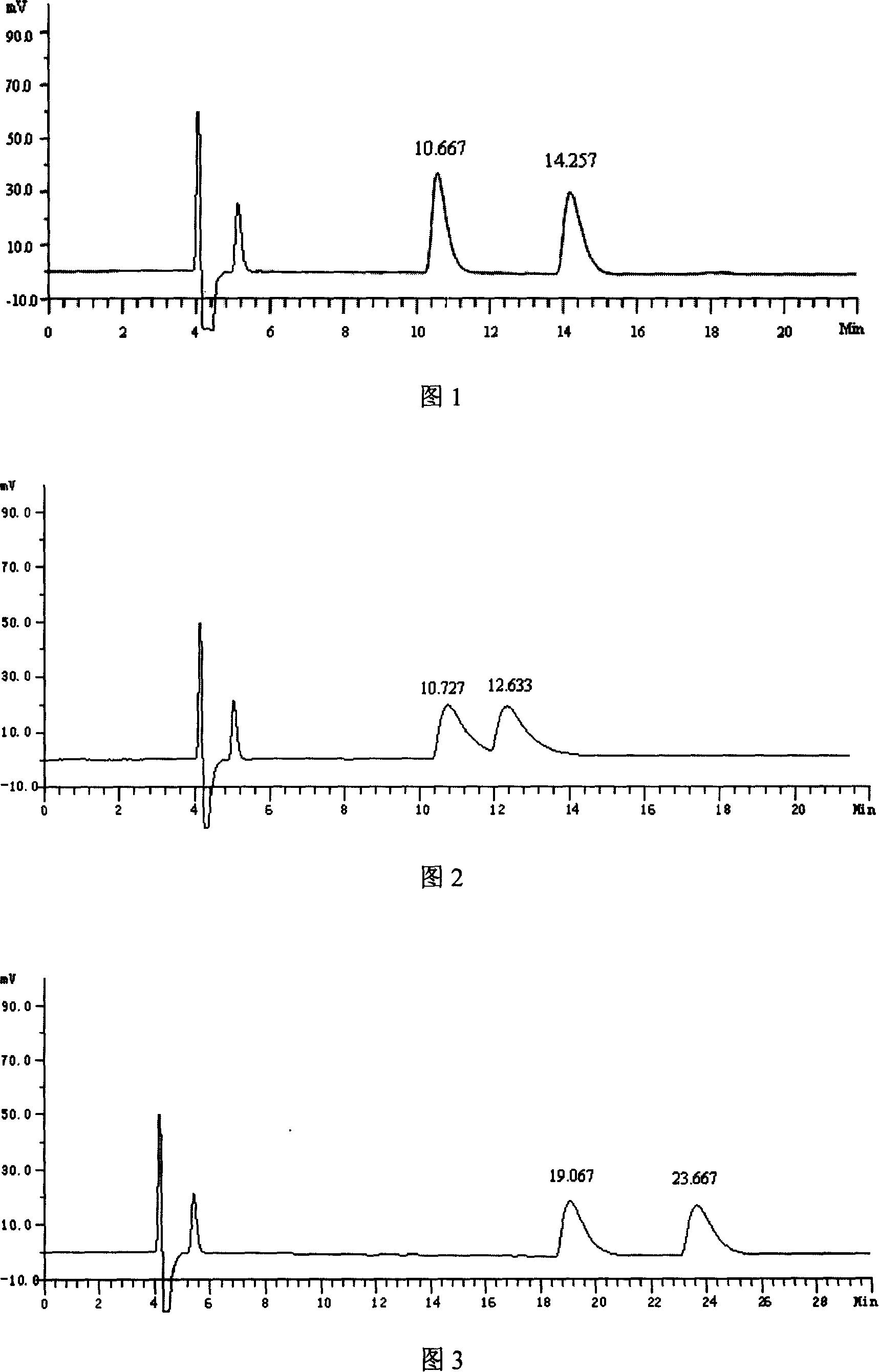
[0026] Instruments and conditions: High performance liquid chromatography: Japan Shimadzu: LC-10Avp, SPD-10Avp; Chromatographic column: OJ-H (250mm×4.6mm) chiral column; Mobile phase: n-hexane-isopropanol-triethyl Amine=97:3:0.5 (V:V:V); flow rate: 0.8 mL / min; detection wavelength: 266 nm; column temperature: room temperature; injection volume: 10 μL.
[0027] Experimental steps:
[0028] (1) Take 10mg of 4-(p-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine racemate, put it in a 10mL volumetric flask, add methanol to dissolve and dilute to the scale, shake well, and use it as the test product solution.
[0029] (2) Take the reagent blank solution and the test solution respectively, carry out high-performance liquid chromatography analysis according to the above conditions, and record the chromatograms.
[0030] The results are shown in accompanying drawing 1, and what retention time is 10.667min is (3S, 4R)-4-(p-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine, and what ret...
Embodiment 2
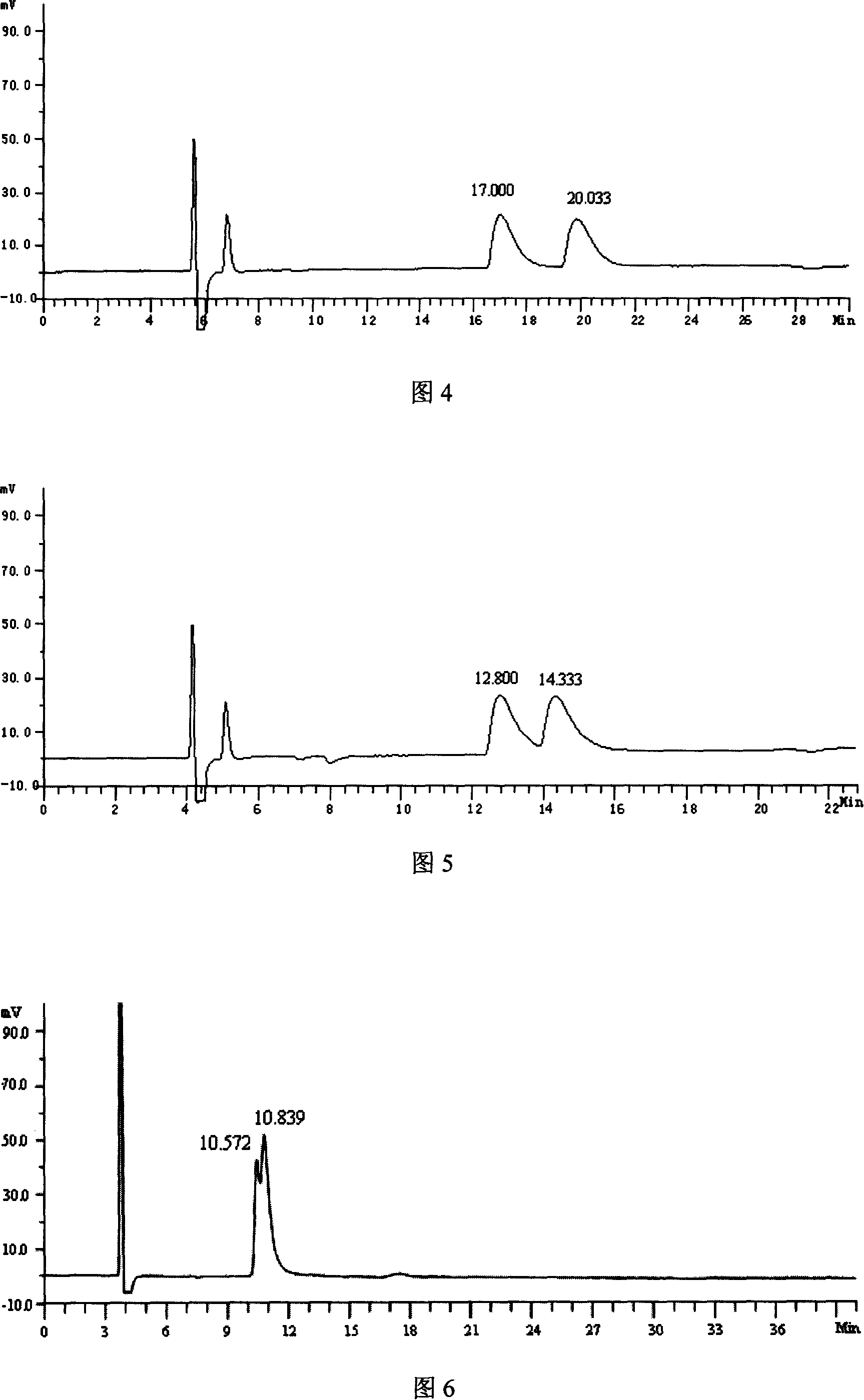
[0032] Instruments and conditions: High performance liquid chromatography: Japan Shimadzu: LC-10Avp, SPD-10Avp; Chromatographic column OD-RH (150mm×4.6mm) chiral column; Mobile phase: n-hexane-isopropanol-triethylamine =97:3:0.5 (V:V:V); flow rate: 0.8 mL / min; detection wavelength: 266 nm; column temperature: room temperature; injection volume: 10 μL.
[0033] Experimental steps:
[0034] (1) Take 10mg of the racemate of 4-(p-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine, put it in a 10mL volumetric flask, add methanol to dissolve and dilute to the scale, shake well, and use it as the Test solution.
[0035] (2) Take the reagent blank solution and the test solution respectively, carry out high-performance liquid chromatography analysis according to the above conditions, and record the chromatograms.
[0036] Result is shown in accompanying drawing 2, and retention time is that (3S, 4R)-4-(p-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine is 10.727min, and retention time ...
Embodiment 3
[0038] Instruments and conditions: High performance liquid chromatography: Japan Shimadzu: LC-10Avp, SPD-10Avp; Chromatographic column: OJ-H (250mm×4.6mm) chiral column; Mobile phase: n-hexane-methanol-triethylamine= 97:3:0.5 (V:V:V); flow rate: 0.8 mL / min; detection wavelength: 266 nm; column temperature: room temperature; injection volume: 10 μL.
[0039] Experimental steps:
[0040] (1) Take 10mg of the racemate of 4-(p-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine, put it in a 10mL volumetric flask, add methanol to dissolve and dilute to the scale, shake well, and use it as the Test solution.
[0041] (2) Take the reagent blank solution and the test solution respectively, carry out high-performance liquid chromatography analysis according to the above conditions, and record the chromatograms.
[0042] The results are shown in Figure 3. What retention time is 19.067min is (3S, 4R)-4-(p-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine, and what retention time is 23.667mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com