Streptavidin-interleukins 2 fusion protein
A technology of interleukin and fusion protein, applied in the field of interleukin-2, which can solve the problems of limited anti-tumor clinical effect, time-consuming preparation of autologous tumor cell vaccine, and difficulty in achieving effective concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
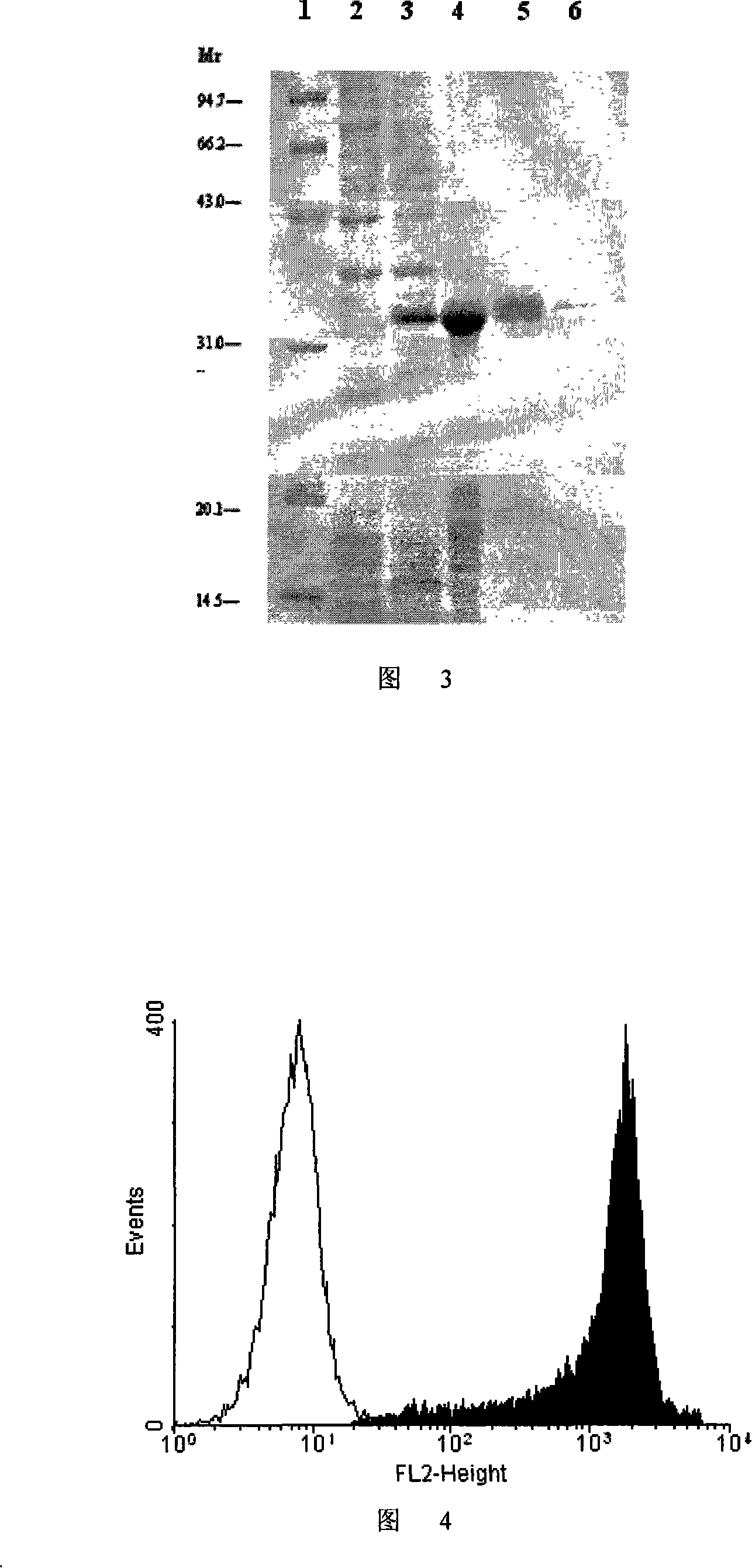
Image
Examples
example 1
[0028] Preparation of Example 1 Fusion Protein IL2-L-SA-6His
[0029] 1. Use the DNeasy tissue kit to extract bacterial genomic DNA from Streptomyces avidin, and then use it as a template to prepare mature streptavidin cDNA by PCR with Platinum pfx DNA polymerase.
[0030] Primer: 5'GGAATTCTCAAGCGGGGGCAGCGGGGGCGGAGGCAGCGGCGGGGGCGGATCCG CCGACCCCTCCAAGGACTCGAAGGCC 3'(78nt) and
[0031] 5'GTGGTGCTCGAGCTGCTGAACGGCGTCGAGCGGGTTGCC 3' (39nt).
[0032]Reaction conditions: denaturation at 94°C, 2min, cycle (94°C, 15s→60°C, 15s→68°C, 30s) for 25 rounds, and finally 68°C, 5min.
[0033] 2. Extract the total RNA of PHA-activated peripheral blood lymphocytes with Trizol, and use it as a template for RT-PCR to prepare mature IL-2 cDNA.
[0034] Primers: 5'CATGCCATGGCTCCTACTTCAAGTTCTACAAAG 3'(33nt) and
[0035] 5'GGAATTCAGTCAGTGTTGAGATGATGCTTTG 3'(31nt)
[0036] Reaction conditions: denaturation at 94°C, 2min, cycle (94°C, 15s→60°C, 15s→68°C, 30s) for 25 rounds, and finally 68°C, 5min. ...
example 2
[0050] Preparation of Example 2 Fusion Protein 6His-SA-L-IL2
[0051] 1. Preparation of mature streptavidin cDNA: the method is the same as in Example 1.
[0052] Primers:
[0053] 5'GGAATTCCATATGCATCATCACCATCACCATGAGGCCGGCATCACCGGCACCTGG3' (55nt) and
[0054] 5' GGAATTCGGCGGATCCGCCCCCGCCGCTGCCTCCGCCCCCGCTGCCCCCGCTCGTCTGCTGAACGGCGTCGAGCGGGTTGCC 3' (82nt).
[0055] 2. Preparation of mature IL-2 cDNA: the method is the same as in Example 1.
[0056] Primers:
[0057] 5'GGAATTCATGGCTCCTACTTCAAGTTCTAC 3'(30nt)
[0058] and 5' CCCAAGCTTTCAAGTCAGTGTTGAGATGATGCTTTG 3' (36nt).
[0059] 3. Construction of 6His-SA-L-IL2-pET24 recombinant plasmid
[0060] Prepared SA cDNA (without termination code, containing NdeI and EcoRI restriction endonuclease sites at both ends) and IL-2 cDNA (respectively containing EcoRI and HindIII restriction endonuclease sites at both ends), the above SA and IL-2 gene fragments were cloned into the pET-24a vector to obtain the 6His-SA-L-IL2-pET24 recomb...
example 3
[0066] Example 3 Preparation of IL2-L-SA-6His or 6His-SA-L-IL2 Fusion Protein Modified Tumor Vaccine
[0067] will be 10 7 Suspend B16.F10 cells in 1ml 1×PBS, add 0.5mg Sulfo-NHS-LC-Biotin and mix well, then act at room temperature for 30 minutes; wash the cells 3 times with 1×PBS, 6 Add 200ng IL2-L-SA-6His or 6His-SA-L-IL2 fusion protein to each B16.F10 cell, and let it act on ice for 30 minutes; wash the cells once with 1×PBS, and then inactivate with γ-rays (20000rad). .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com