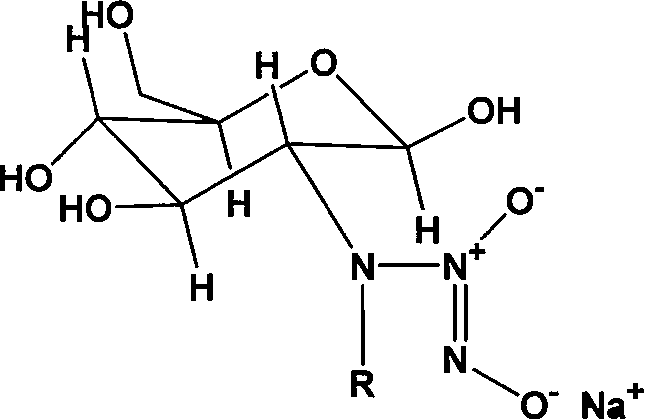
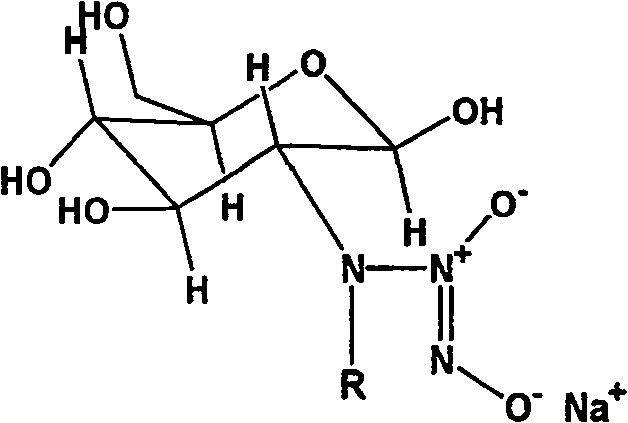
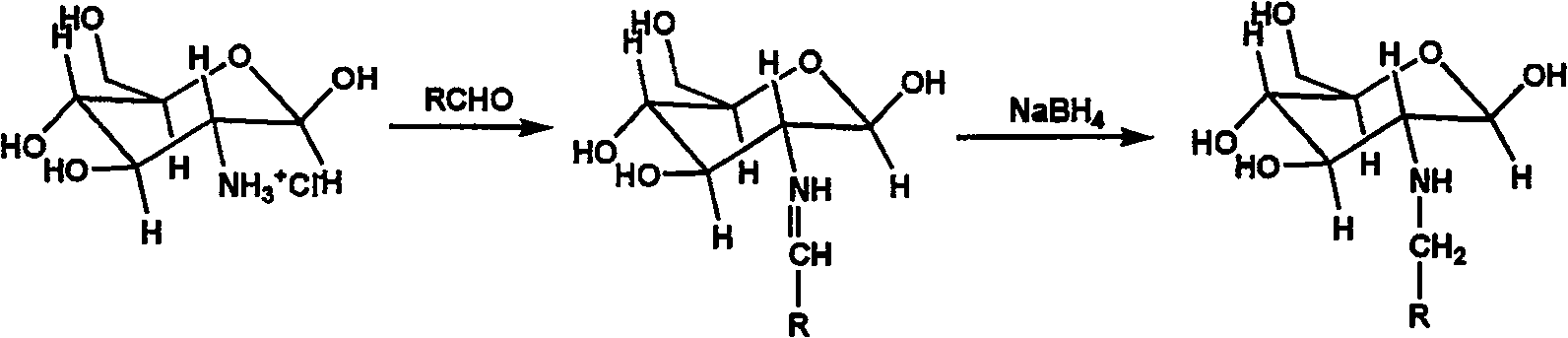
Nucleophilic N0 donor of alkyl modified amido glucose, and synthetic method
A technology of glucosamine and alkyl modification, which is applied in the field of medical engineering, can solve the problems of insufficient NO response, low NO load, and low NO load, so as to improve antithrombotic performance, promote wound healing, and prevent restenosis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of propionaldehyde (PA) modified glucosamine / NO
[0026] Add 10g (0.0506mol) of glucosamine hydrochloride (GS) into 100ml of methanol solution, add 50ml of water to dissolve it, add 5.5ml of propionaldehyde (GS:PA molar ratio 1:1.5) into the system, and react at room temperature for 12h , 3.83 383g (0.1012mol, GS: NaBH 4 The molar ratio of = 1:2) NaBH 4 Dissolve in 25ml of water, slowly drop into the solution, reduce for 12 hours to obtain a light yellow solution, use 100ml of acetone to precipitate viscous substances, filter, and the filtrate is rotary evaporated, and the obtained solid is heated and dissolved with methanol, then filtered, and the product is purified with methanol repeatedly , and finally dried to obtain 5 g of light yellow crystal powder.
[0027] Add 2.5g (0.01mol) of the above reaction product to 100ml of anhydrous methanol solution containing 2.15g (0.02mol) sodium methoxide, react with NO in an autoclave, maintain the...
Embodiment 2
[0029] Embodiment 2: the synthesis of glutaraldehyde modified glucosamine hydrochloride / NO
[0030] 10g (0.0506mol) glucosamine hydrochloride (GS) was added in the methanol solution of 100ml, 20.24g 25% glutaraldehyde aqueous solution (GS: the molar ratio of glutaraldehyde=1: 1) was added in the solution, room temperature reaction 24h. 3.83g (0.1012mol, GS: NaBH 4 The molar ratio of = 1:2) NaBH 4Dissolve in 25ml of water, slowly drop into the solution, reduction reaction 12h. After the reaction is completed, add 30ml of water to the reaction solution, place it in a water bath at 50-60°C and stir for a period of time, the solution will gradually become transparent, and a white sticky precipitate will form when placed at room temperature, filter through filter paper, add 30ml of acetone to the filtrate, and dry the filtrate at 60°C Dry to give 8.1210 g of yellow powder. The product was dissolved in methanol and filtered, and the filtrate was dried to obtain 7.0 g of light ye...
Embodiment 3
[0033] Embodiment 3: the synthesis of n-chlorobutane modified glucosamine hydrochloride / NO donor
[0034] 10g (0.0506mol) glucosamine hydrochloride (GS) was added to 100ml of 10% isopropanol aqueous solution, stirred at 70°C for 30 minutes, and chlorobutane was added dropwise, wherein halogenated hydrocarbon: glucosamine salt The molar ratio of acid salt is 1:1-1.2, react at room temperature for 12 hours, neutralize the solution with hydrochloric acid to neutrality, and rotate to evaporate. About 5g of light yellow crystal powder.
[0035] Add 1 g (0.004 mol) of the above reaction product into 100 ml of anhydrous methanol solution containing 0.86 g (0.008 mol) of sodium methylate, react with NO in an autoclave, maintain the pressure at 5 atm, and react for 3 days. Washed with water, methanol and ether, and dried under vacuum at room temperature to obtain about 1 g of white fluffy powder.
[0036] Determination of [N(O)NO] by UV Spectroscopy - The specific absorption of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com