Biodegradable medical device for breast reconstruction and/or augmentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of PUUEE Foam
[0086]Polyol solution are prepared by mixing the following ingredients as indicated in table 2a, 2b and 2c. Hardener / catalyst solution is prepared as indicated in table 3a, 3b and 3c.
[0087]The polyol and hardener solution are mixed, by means of mechanical stirring at 400-600 rpm, from one to two minutes and let to expand freely for another minute, before solidification.
[0088]According to this procedure, the average pore size of the foam is inversely proportional to stirring time, before cross-linking. The longer the time of mechanical stirring the smaller the pore size. Temperature can accelerate the reaction kinetics, and can be applied to shorten cross-linking time intervals. However according to this casting strategy, which is “one shot”, the exothermic process characterizing the poly addition reaction between the low molecular weight molecules, involved in the formulation, is sufficient to push the conversion degree of the starting materials up to 100%.
TAB...
example 2
Shaping and Channelization of PUUEE Foam
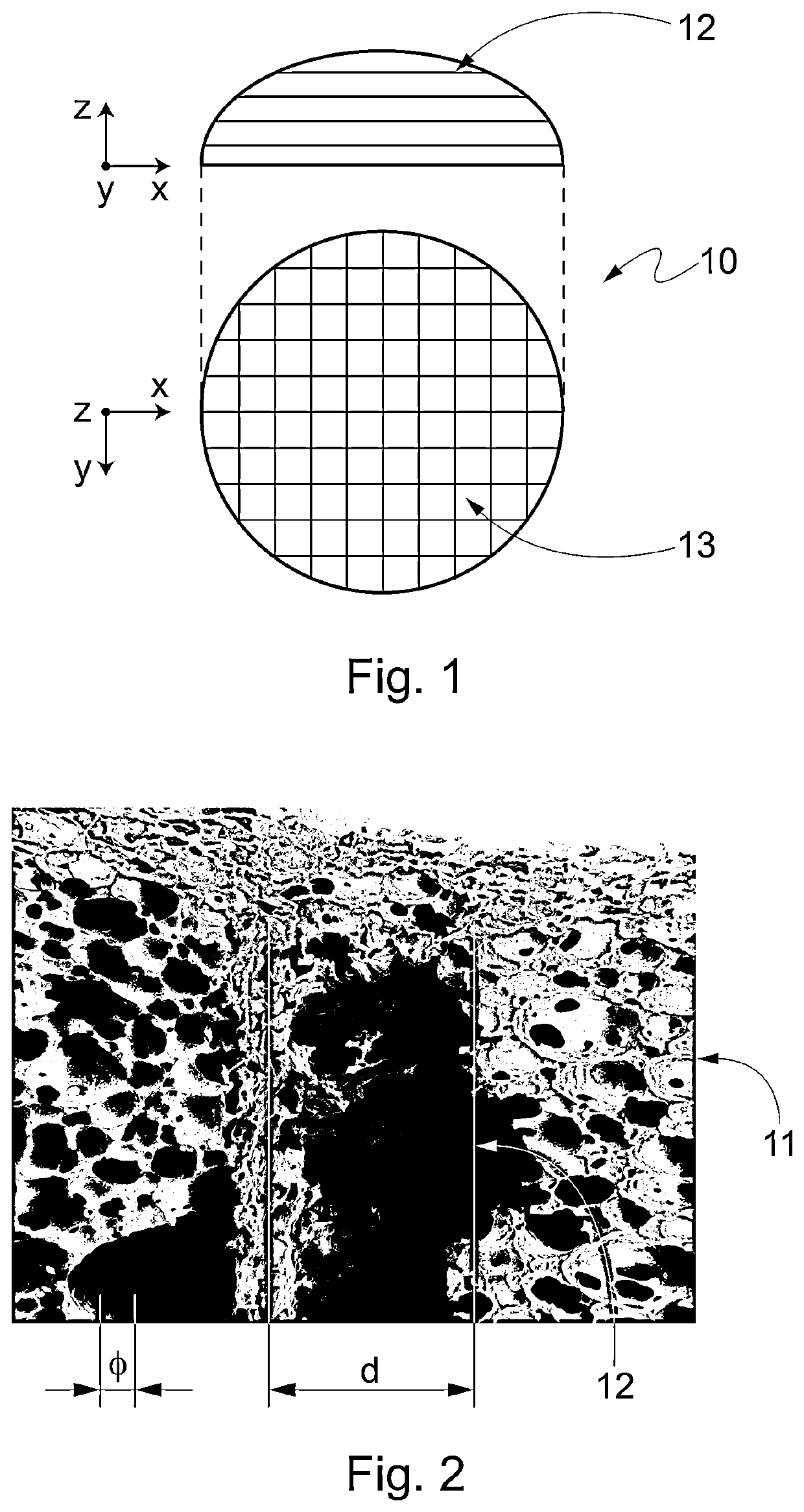
[0092]A PUUEE foam with cylindrical shape is synthesized according to example 1.
[0093]A hot wire is used to cut the external part of the cylinder, in order to obtain a semi-spherical caps.
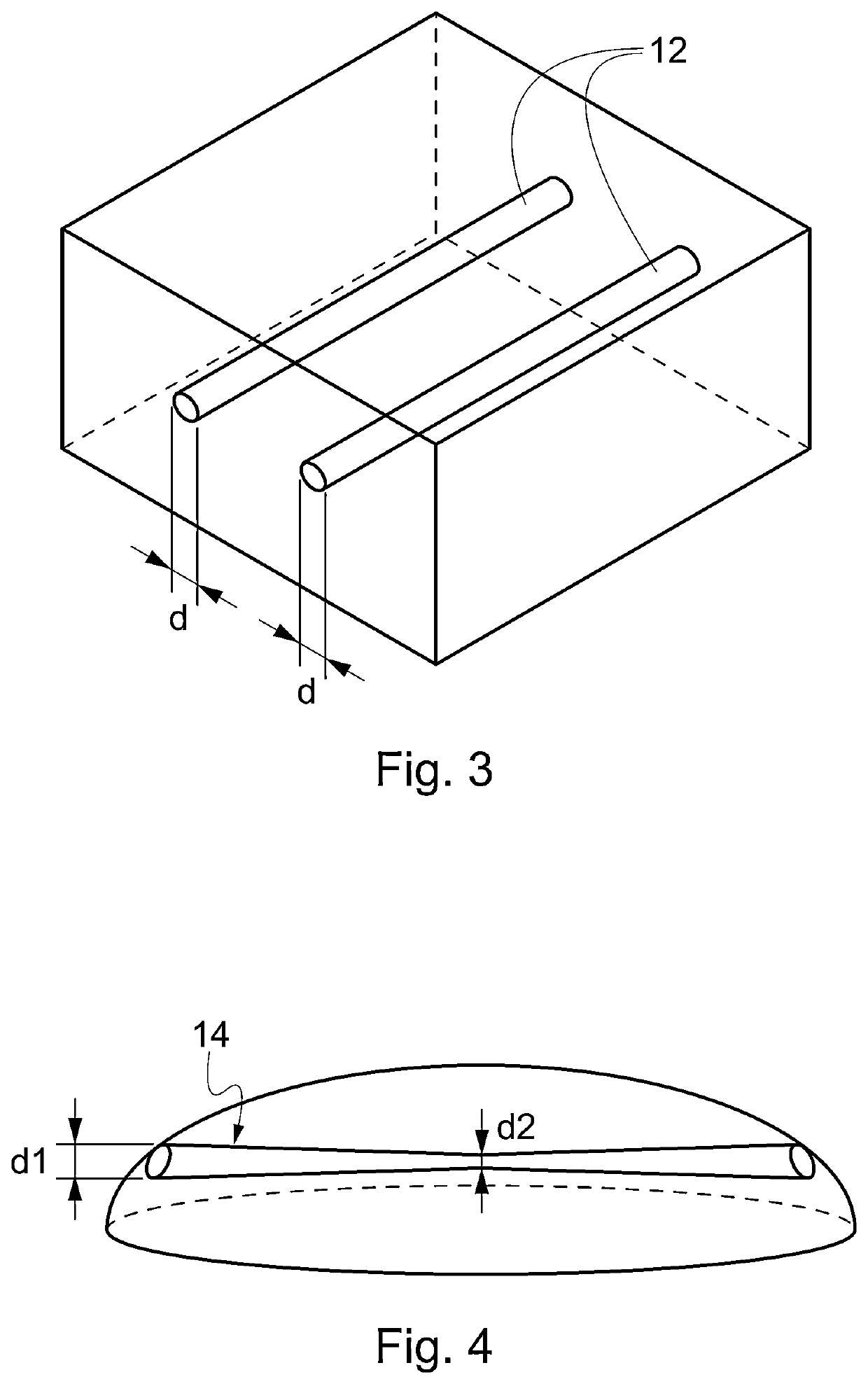
[0094]Once the foam has been shaped externally, a network of channels is realized inside the foam by perforating the foam with a series of hot needles. The needles are heated, electrically, at temperatures range 100-200° C., preferably between 130 and 170° C.
[0095]The needles are inserted through the foam's matrix and retracted after a time interval ranging from 1 second and 20 seconds, preferably between 5 and 10 seconds.
[0096]The diameter of the needle ranges 0.1 mm to 5 mm, preferably between 0.5 and 2 mm. The diameter of the resulting channels depends on both the diameter of the tool and its permanence time inside the foam, where higher permanence times produce larger channels.
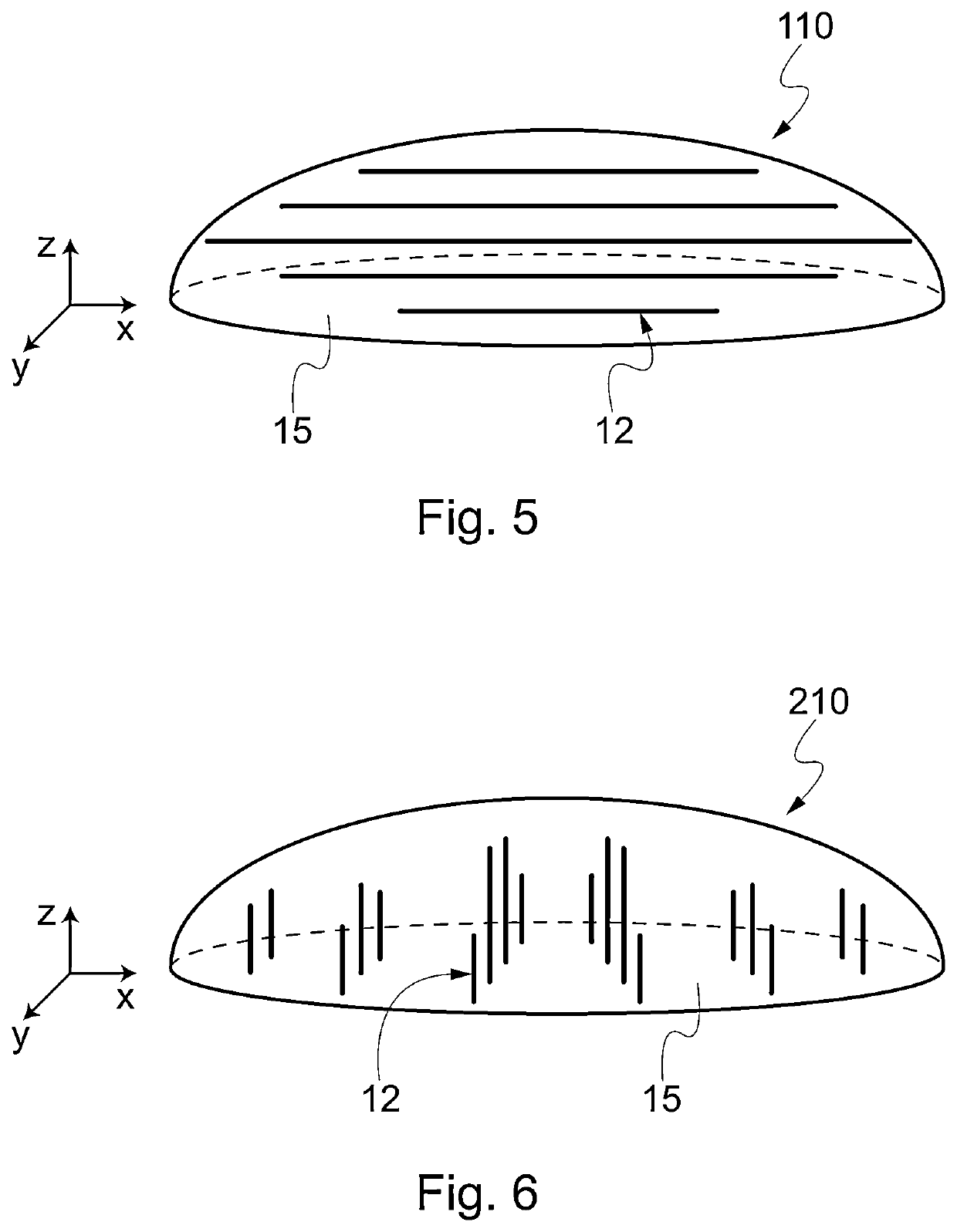
[0097]The network of channels is produced by perforating the foam in different ...
example 3
Chemical and Physical Characterization of PUUEE Prosthesis
[0099]Uniaxial compression test: six cylindrical specimens, of 1 cm diameter and 1 cm height were used to determine the elastic compressive modulus and compressive strength of the prosthesis.
[0100]All measurements were carried out at room temperature (25° C.) on swollen samples in distilled water. The samples were compressed at the speed of 1 mm / min.
[0101]Compressive elastic modulus was calculated by the slope of stress-strain curve at the deformation zone between 5 and 10%.
[0102]Compressive strength was calculated as the maximum stress at deformation higher than 95%.
[0103]The stress strain curve of the prosthesis obtained according to Example 1 is illustrated in FIG. 13.
[0104]The prosthesis are characterized by a 30 kPa compressive elastic modulus and a compressive strength (at 95% deformation) of 1 MPa.
Weight Water uptake tests: six purified and dried cylindrical samples were incubated in phosphate buffer saline solution (P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com