Fourier ptychographic microscopy with multiplexed illumination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
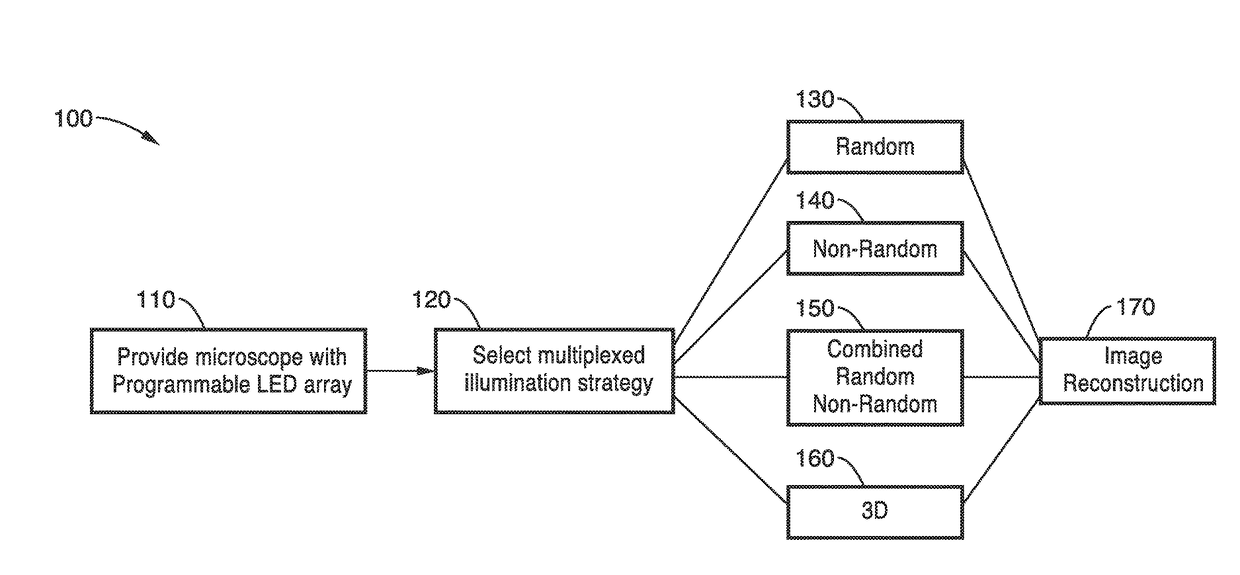
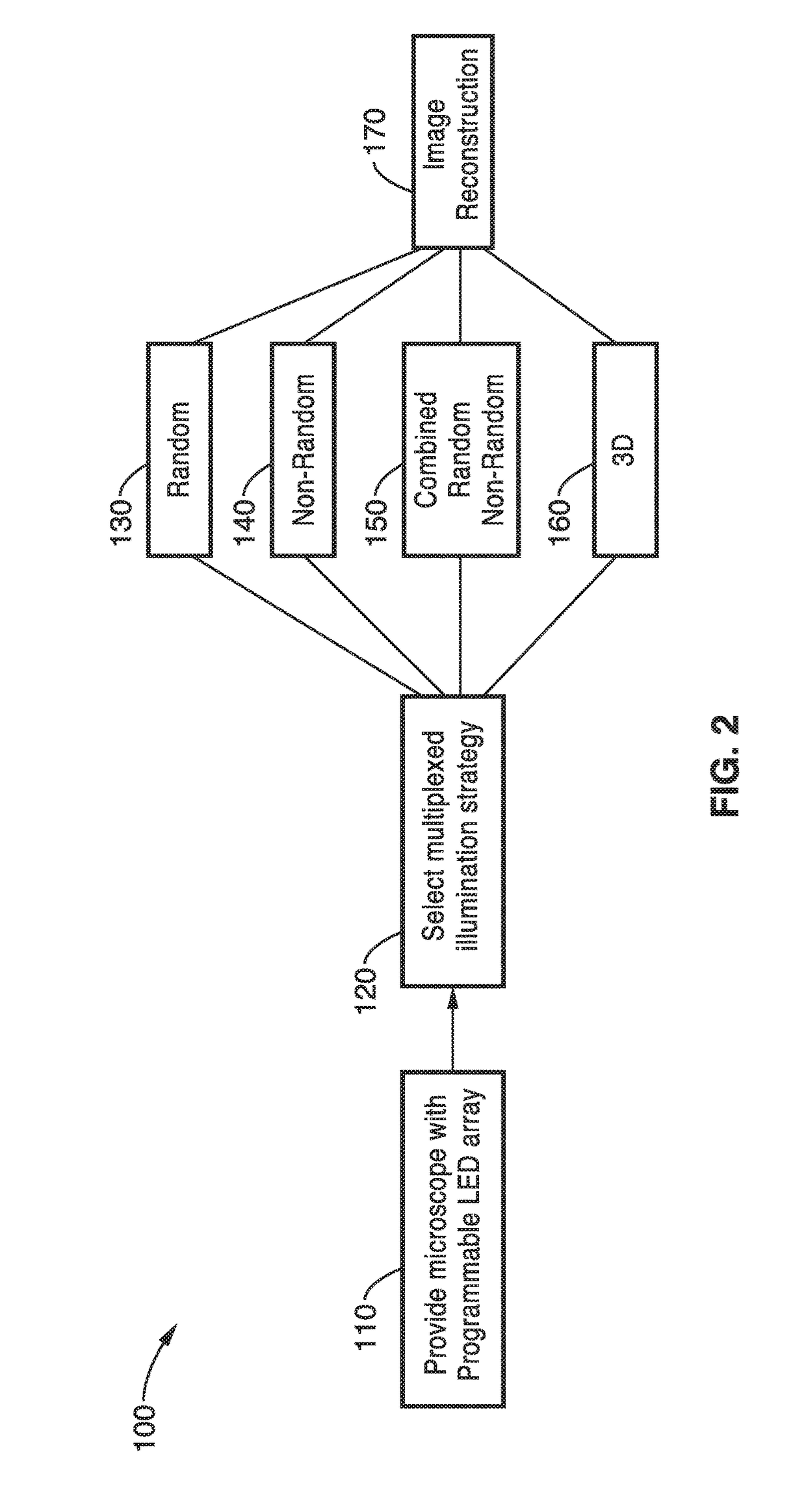
Method used
Image
Examples
example 1
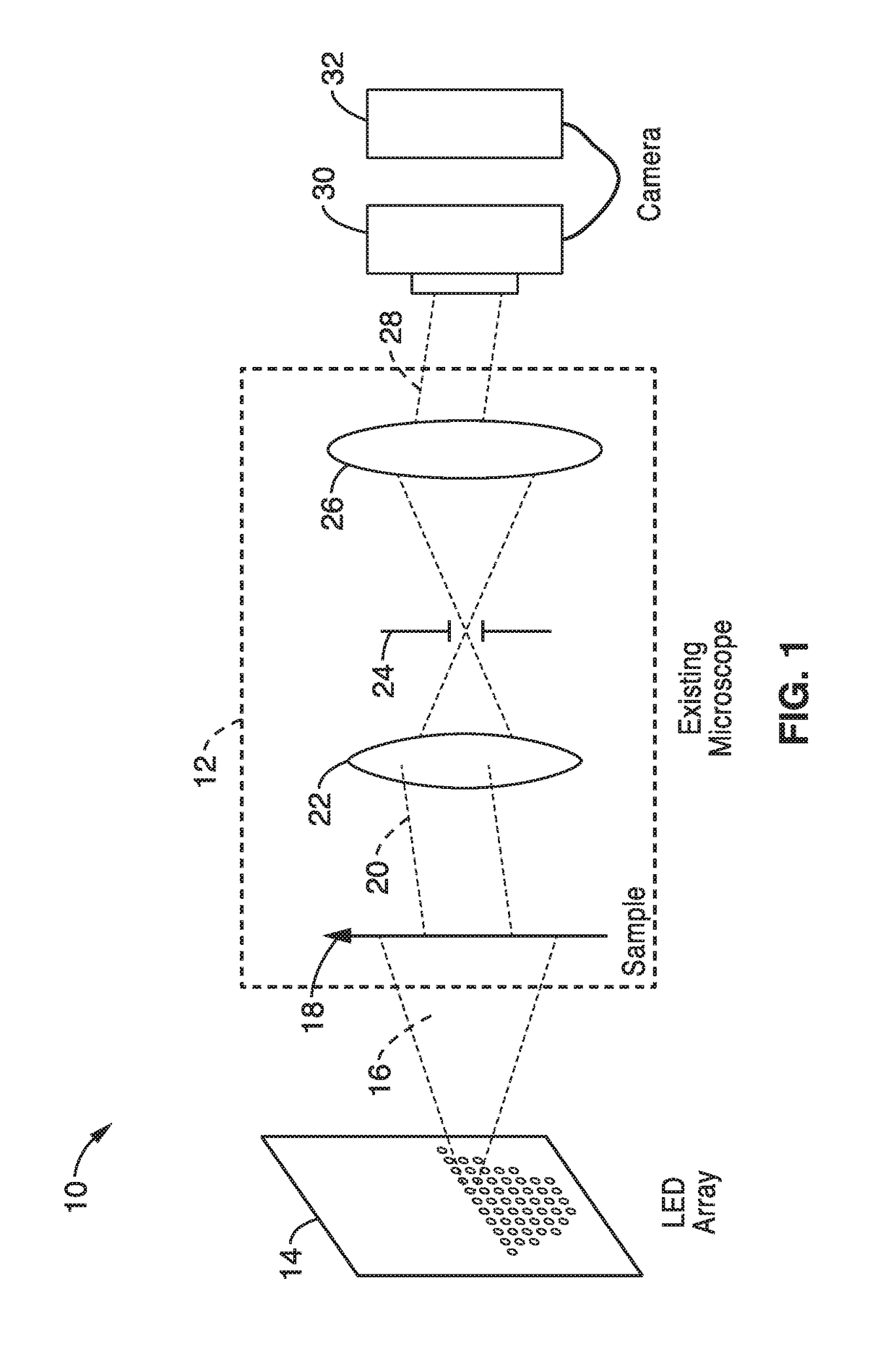
[0113]In order to demonstrate a random multiplexed illumination strategy, where multiple randomly selected LEDs are turned on for each image, an apparatus as schematically shown in FIG. 1 with a programmable LED array was assembled. All samples were imaged with a 4×0.1 NA objective and a scientific CMOS camera. A programmable 32×32 LED array (Adafruit, 4 mm spacing, controlled by an Arduino) was placed at 67.5 mm above the sample to replace the light source on a Nikon TE300 inverted microscope. The central 293 red (central wavelength 629 nm and 20 nm bandwidth) LEDs were used resulting in a final synthetic NA of 0.6. In principle, the LED array could provide larger NA improvements, but it is practically limited by noise in the dark field images from high angle LEDs.
[0114]Since each LED corresponds to a different area of Fourier space, the total number of images can be significantly reduced, without sacrificing image quality. The method was demonstrated experimentally in a modified c...
example 2
[0121]To demonstrate the non-random illumination methods, an LED array microscope with a 32×32 custom-made LED array (4 mm spacing, central wavelength 513 nm with 20 nm bandwidth) placed 63 mm above the sample was used to replace the microscope's standard illumination unit. The LED array was controlled by an ARM microcontroller and synchronized with the camera to switch the LED patterns at camera-limited speed. The camera operated at 50 Hz with full-frame (2160×2560) 16 bit images allowing single-axis DPC measurements at 25 Hz. Example images all used Hela cell samples imaged by a 20×0.4 NA objective.
[0122]The phase transfer function can be tuned by using different illumination patterns. DPC is commonly implemented with half-circle shape illumination patterns. DPC requires illumination from high angles for good phase recovery at all spatial frequencies. As the illumination NA increases (measured by the coherence parameter a), additional low spatial frequency and high spatial frequen...
example 3
[0127]To further demonstrate the operational principles of the methods, a system of an inverted microscope with a programmable 32×32 individually addressable LED array to pattern illumination angles was assembled.
[0128]All LEDs were driven statically using 64 LED controller chips (MBI5041) to provide an independent drive channel for each LED. A controller unit based on the ARM 32-bit Cortex M3 CPU provides the logical control for the LED array by the I2C interface at 5 MHz. The LED pattern transfer time is about 320 ps. A standard stage-mounted incubator was used for imaging live cell samples in a petri dish.
[0129]For specimens, HeLa cells, Human osteosarcoma epithelial (U2OS) cells and human mammary epithelial (MCF10A) cells were cultured with DMEM (Dulbeccos Modified Eagles Medium) supplemented with 10 % FBS (fetal bovine serum), Glutamine and penicillin / streptomycin. The cells were plated on p75 flasks and cultured in 37 degree incubator with 5% CO2.
[0130]The random, non-random a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com