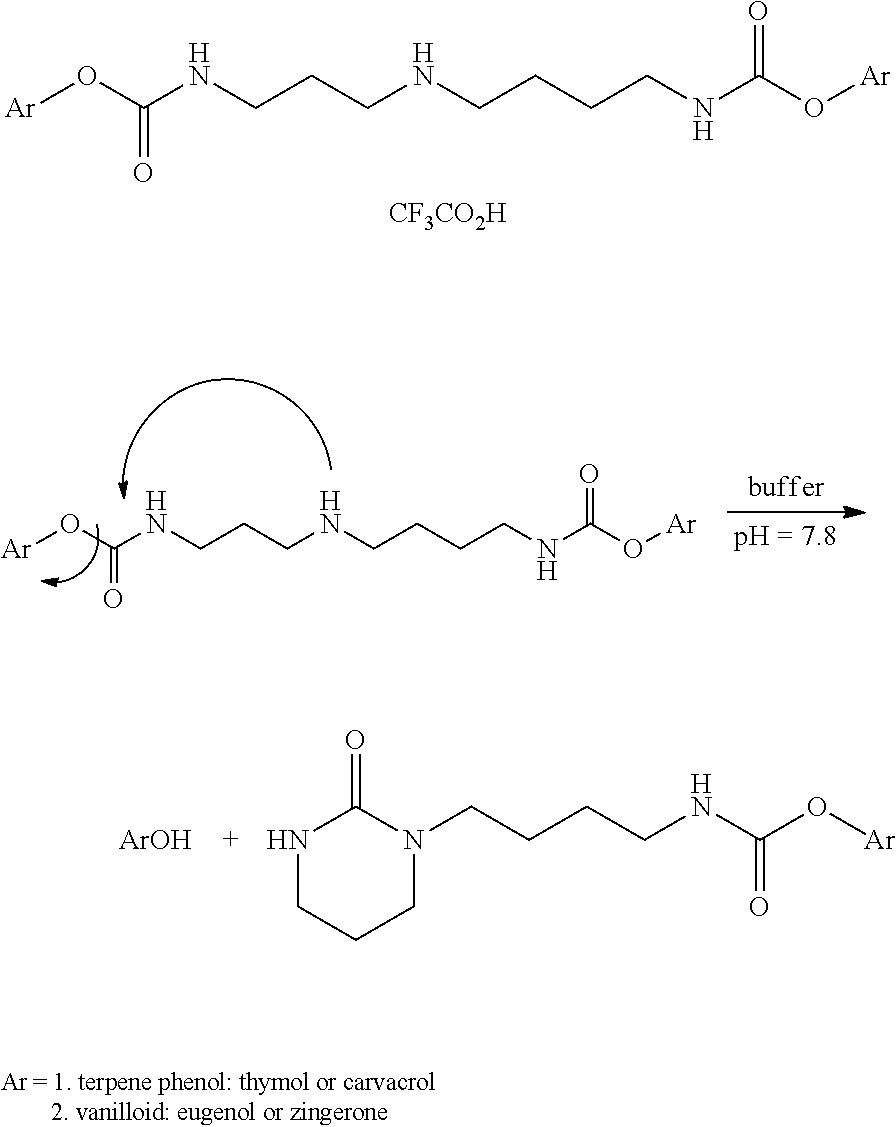
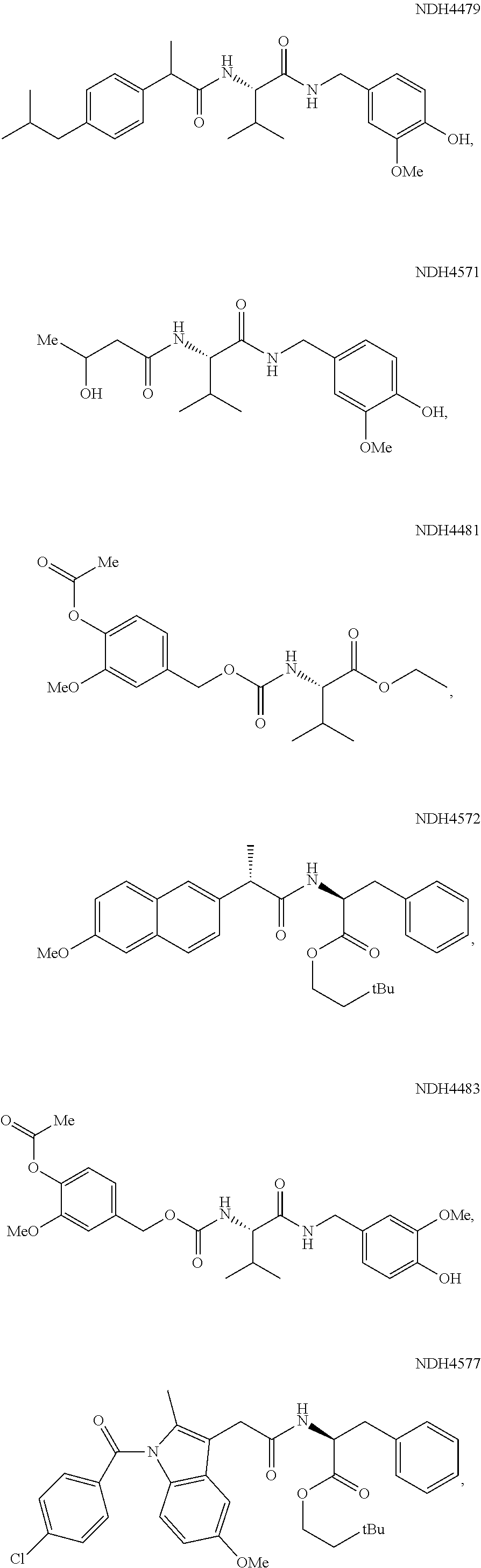
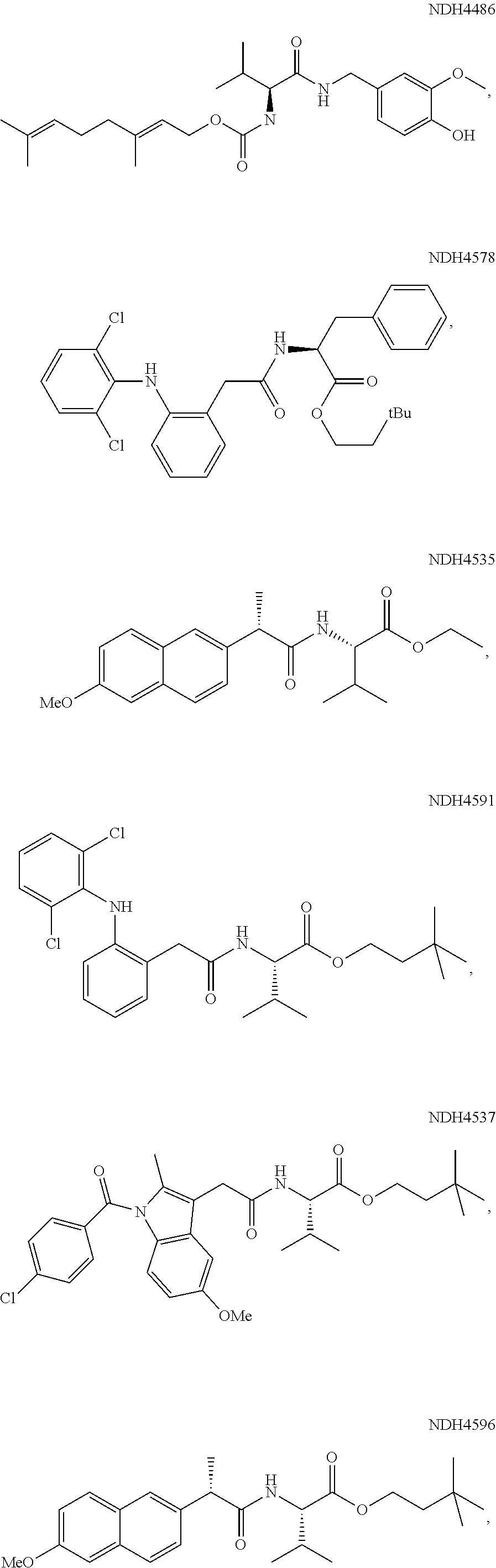
Augmenting Moieties for Anti-Inflammatory Compounds
a technology of anti-inflammatory compounds and moieties, which is applied in the field of anti-inflammatory compounds, can solve the problems of ulcer perforation, death, and limiting the use of nsaid therapy, and achieve the effect of improving the potency and safety of anti-inflammatory compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
[0050]All reactants and solvents used were of the highest purity commercial grade and were employed without further purification. All amino acids used herein were the L-amino acids and were purchased from Sigma-Aldrich (Saint Louis, Mo.). The 2-(2-methoxynaphthalene-6-yl) propanoic acid (naproxen) used was the (S)-enantiomer. All other reagents were used as racemates, unless otherwise noted. All reactions were performed in oven-dried apparatus under a nitrogen atmosphere, unless otherwise noted. All solvents used were anhydrous, unless otherwise noted. NMR spectra were recorded on a Bruker multinuclear spectrometer and chemical shifts are reported as ppm using tetramethylsilane (TMS) as an internal standard. 1H NMR spectra were recorded at 500 MHz, while 13C NMR spectra were recorded at 125 MHz. Elemental analyses were performed at Intertek (Whitehouse, N.J.). All thin layer chromatography (TLC) was performed on Analtech silica gel plates (250 microns).
Biologica...
examples of aspect i
[0054]The bifunctional and tri-functional conjugates of Aspect I of the invention were prepared and tested in a standard in vivo MEVM assay for their efficacy compared to that of the parent terpene, amino acid, polyamine, or vanilloid from which each was assembled. Terpene inflammation suppression scores (average of TPA-induced and CEES-induced injuries) ranged from myrtenol (6%), thymol (14%), carvacrol (15%), cumyl alcohol (16%), geraniol (35%), menthol (38%), perillyl alcohol (43%), and farnesol (69%). All terpenes, except farnesol, had inflammation suppression scores less than 45%. The inflammation scores of typical vanilloids were similarly low and none exceeded 40%, e.g., vanillin (11%), vanillyl alcohol (31%), and vanillylamine (35%). In this assay inflammation suppression scores for the amino acids and the polyamines were under 30%. The traditional NSAIDs were under 40% in inflammation suppression scores, e.g., ibuprofen (−23%, an irritant / inflammation inducer), S-naproxen (...
examples of aspect ii
[0118]Synergism of anti-inflammatory responses by anti-inflammatory agents covalently coupled to amino acids (Aspect II) was demonstrated by preparation of the S-naproxen-valine conjugate, and screening it in the MEVM against CEES challenge. MEVM is a standard in vivo assay for assessment of anti-inflammatory potential in addressing chemically-induced injury to rodent skin. CEES is one of the inflammation inducers employed in the MEVM assay. A compound of the invention, Formula (IV-acid) (NDH 4476) provided four times better inflammation suppression (44%) than naproxen itself under the same conditions. The corresponding ethyl ester analog (IV-ethyl ester) (NDH 4535) was equipotent but the 3.3-dimethylbutyl ester (IV-3,3-dimethylbutyl-) (NDH 4596) was superior at 52% inflammation suppression. The latter molecule also was an inhibitor of AChE displaying anti-cholinergic activity with an IC50 of 18.6 μM.
[0119]The phenylalanine conjugate of S-naproxen (esterified as the 3,3-dimethylbuty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com