Methods for Purifying Polysaccharides and Pharmaceutical Compositions and Medical Devices Containing the Same
a polysaccharide and composition technology, applied in the field of natural-occurring materials purification, can solve the problems of inability to remove endotoxins, inability to perform a relatively short period of time, and inability to achieve the effect of reducing the risk of endotoxins, cost-effective, and convenient to us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
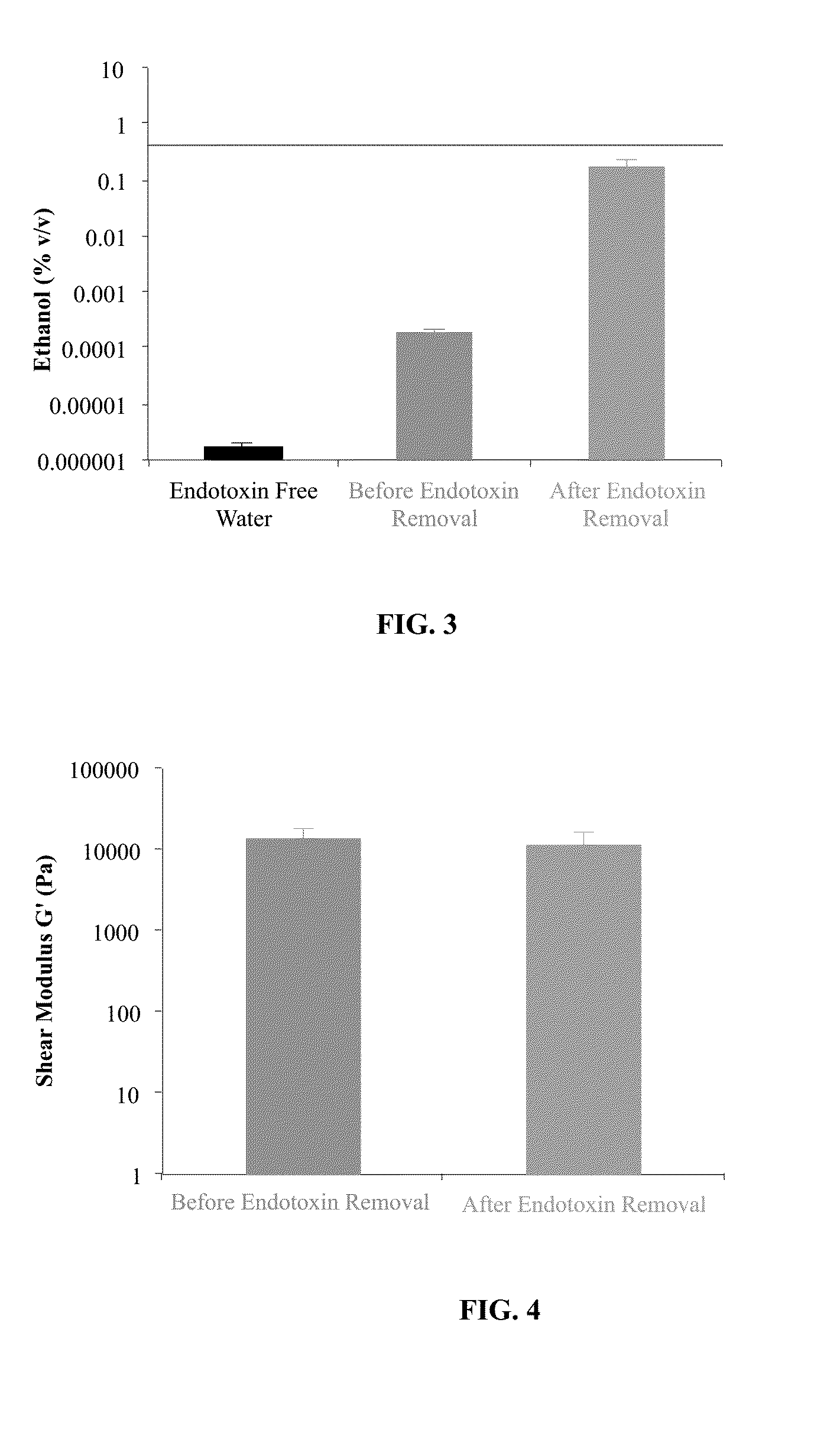
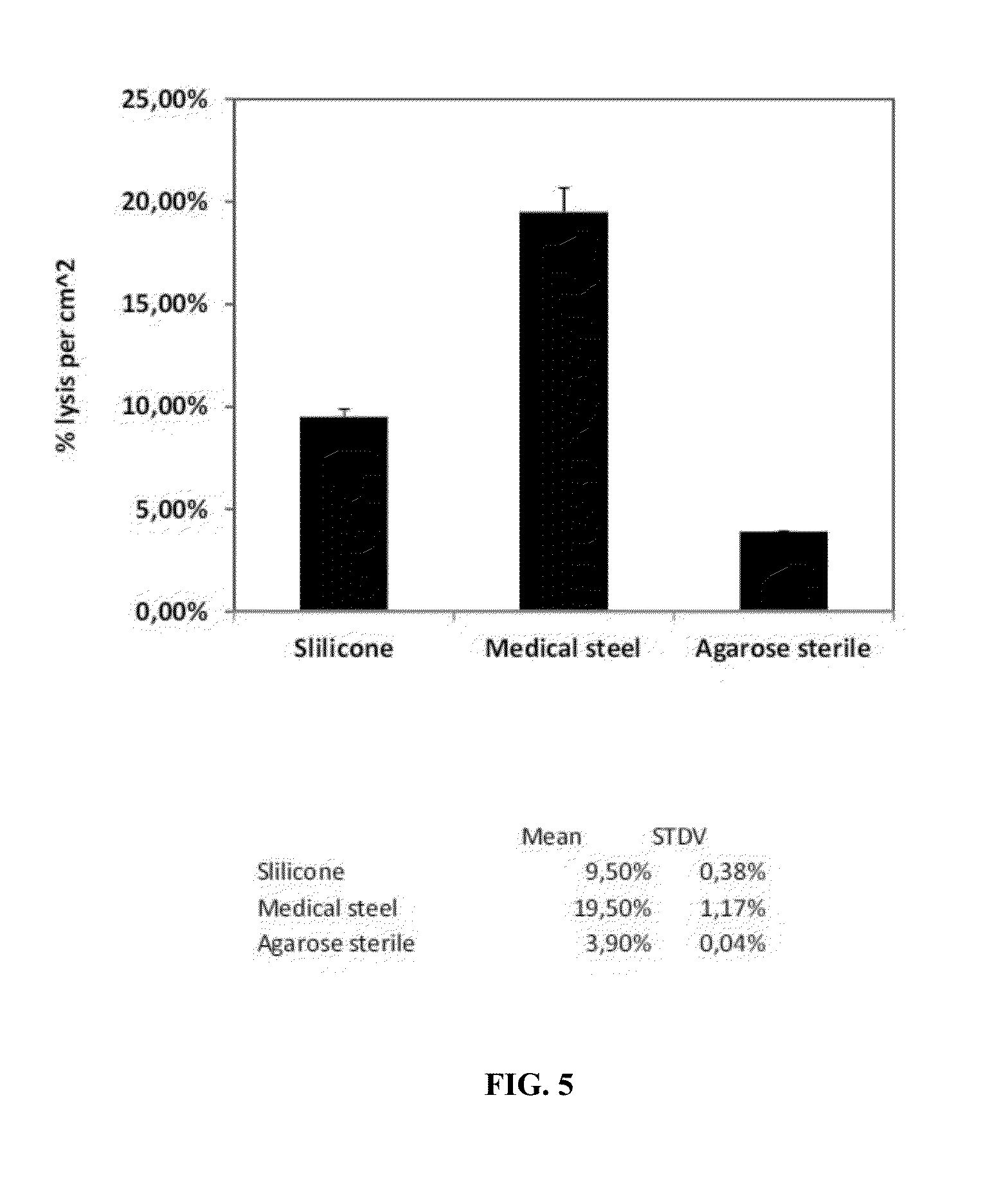
Purification of Polysaccharide and Removal of Bacterial Lipopolysaccharide
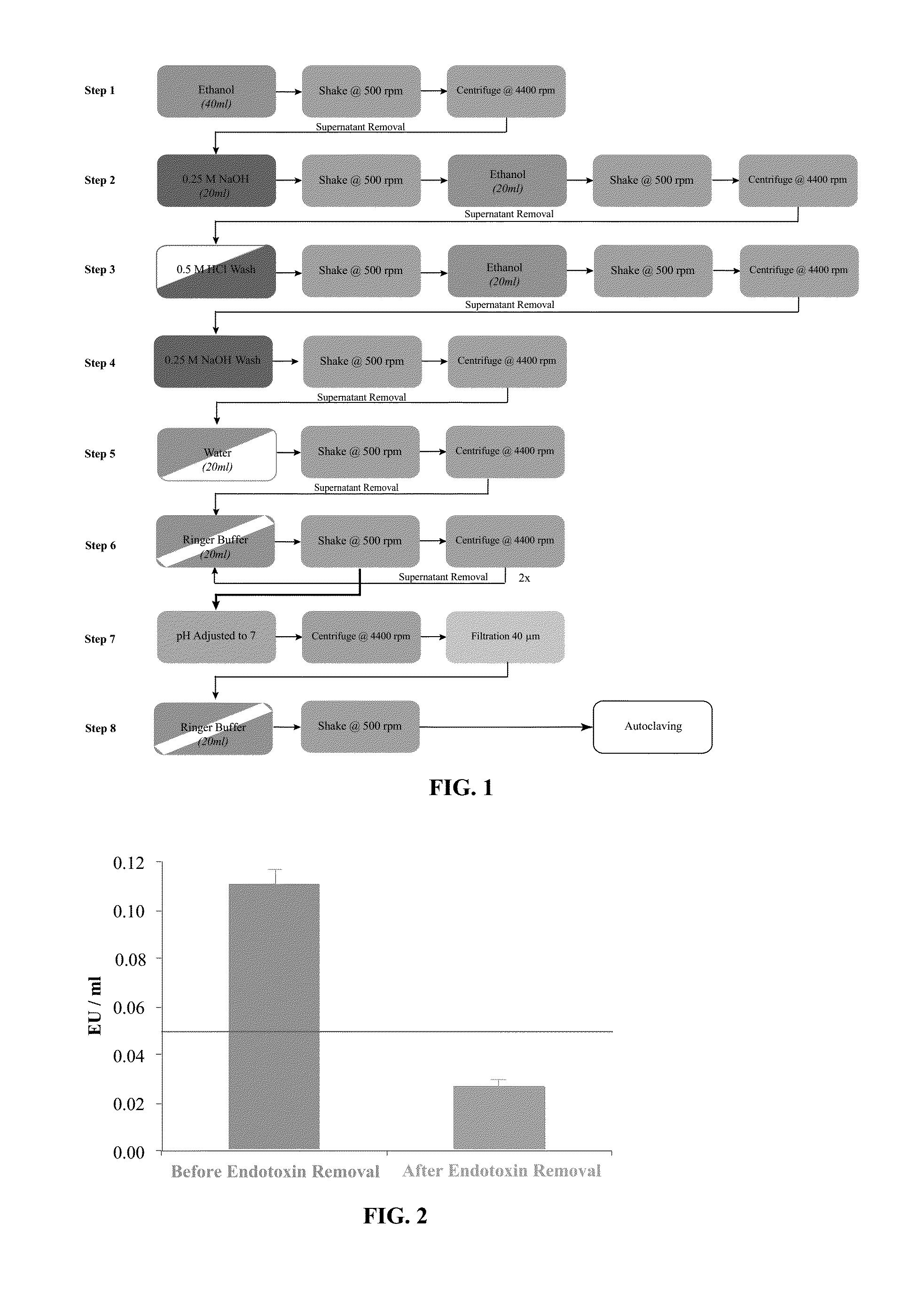
[0085]The procedure was performed under a laminar flow hood that was previously cleaned with an antibacterial / antiviral solution followed by a 1 hour UV light exposure. The operator wore gloves and protective gear required for chemical and biological work to avoid product contamination and to ensure personal safety. All the solutions used were pharmaceutical grade and all tools, which were in direct contact with the product or the reagents were sterile and specified endotoxin free. A flow chart of the isolation and purification process is shown in FIG. 1.
[0086]The procedure for isolating and purifying agarose was conducted as follows:
[0087]Step 1: In order to disrupt the bacteria wall and solubilize the lipid part of the endotoxin, the agarose was incubated under agitation for two minutes in ethanol. Agarose powder was recovered by centrifugation and removal of the supernatant.
[0088]Step 2: A 0.25M solution of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com