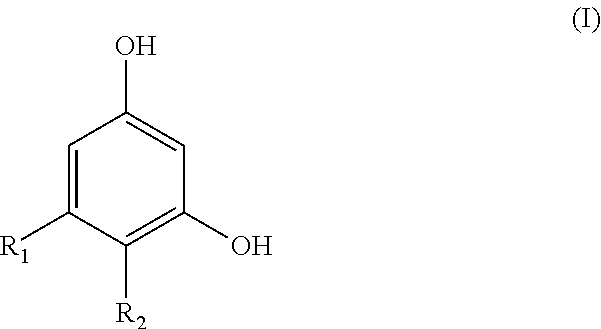
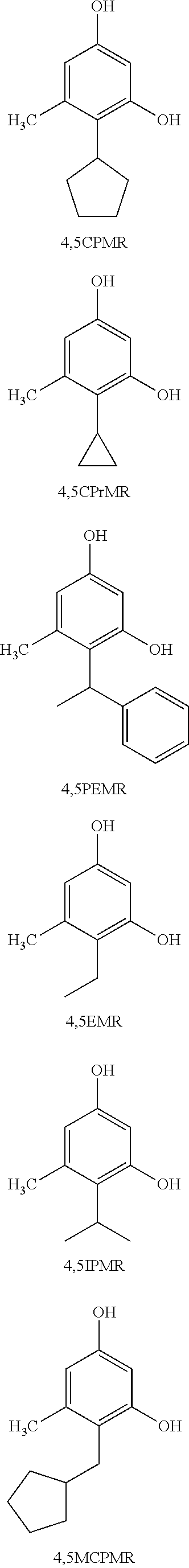
Resorcinol compounds for dermatological use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
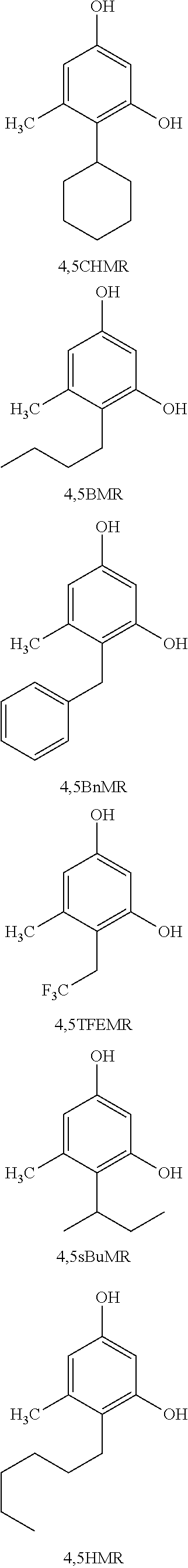
4-cyclohexyl-5-methylbenzene-1,3-diol (4,5CHMR)
[0122]
[0123]4-bromo-1,5-bis(methoxymethoxy)-3-methylbenzene. 26.10 g of orcinol and 19.10 g of ammonium bromide were weighed into a 2000 mL 3-neck round bottom flask fitted with a stir bar, thermometer, and nitrogen bubbler. A nitrogen atmosphere was established and maintained. 1000 mL of acetonitrile was added, and the mixture was stirred rapidly to suspend the solids. The suspension was cooled to 10° in an ice bath and 67.88 g of Oxone™ (OXONE is a registered trademark of DuPont for a monopersulfate oxidizing compound) was added in one portion. The ice bath was removed, and the mixture was stirred at 20° for 30 h. The solids were removed by filtration. The solvent was removed from the filtrate to obtain a dark orange solid. The solid was partitioned between 200 mL of 1.0 M hydrochloric acid and 400 mL tert-butyl methyl ether. The phases were separated, and the aqueous phase was extracted with 200 mL tert-butyl methyl ether. The combin...
example 2
4-cyclohexyl-5-fluorobenzene-1,3-diol (4,5CHFR)
[0127]
2-bromo-1-fluoro-3,5-dimethoxybenzene
[0128]28.22 g of 3,5-dimethoxy-5-fluorobenzene was weighed into a 1,000 mL round bottom flask fitted with a stir bar, reflux condenser, nitrogen inlet, and cap. A nitrogen atmosphere was established and maintained throughout the reaction. 200 mL of anhydrous CCl4 was added, followed by 32.61 g of N-bromosuccinimide (NBS). The remaining NBS powder residue was washed into the flask with 100 mL of CCl4. The reaction mixture was stirred at reflux under N, for 4 h, during which time the suspended yellow solids changed color to white. At completion, the precipitated succinimide solids were filtered off and washed thoroughly with 200 mL heptane. The CCl4 was removed from the filtrate by rotary evaporation at 50° C. More succinimide precipitated from the heptane solution and was removed by filtration while the solution was still warm. The remaining heptane was removed in vacuo to obtain a clear, amber ...
example 3
2-Ethyl-1,5-dihydroxy-3-trifluoromethyl-benzene (4,5 ETFMR)
[0133]
1-(2-Iodo-4,6-dimethoxy-phenyl)-ethanone
[0134]To a solution of acetyl chloride (3.12 g, 39 mmol) in dichloromethane (100 mL) was added AlCl3 (6.34 g, 47 mmol) at 0° C. over 30 min. 1-Iodo-3,5-dimethoxy-benzene (10.5 g, 39 mmol) was added to the mixture. The reaction mixture was stirred at room temperature for 1 h. Then ice water (60 mL) was added. The aqueous layer was extracted with dichloromethane (2×30 mL), dried over sodium sulfate and concentrated, and purified by column chromatography (eluted with petroleum ether / ethyl acetate=20:1˜1:1), to give the title compound (6 g, 50%).
1-(2,4-Dimethoxy-6-trifluoromethyl-phenyl)-ethanone
[0135]A mixture of 1-(2-iodo-4,6-dimethoxy-phenyl)-ethanone (3.17 g, 10.1 mmol), difluoro-fluorosulfonyl-acetic acid methyl ester (7.9 g, 41.1 mmol) and CuI (3.8 g, 20 mmol) in NMP (50 mL) was heated at 120° C. under nitrogen overnight. The mixture was filtered and diluted with water (200 mL)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Stress optical coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com