Polymer templated nanowire catalysts
a nanowire catalyst and polymer technology, applied in metal/metal-oxide/metal-hydroxide catalysts, lanthanide oxides/hydroxides, bulk chemical production, etc., can solve the problem of reducing the yield of undesired side products, affecting the adsorption rate of reactants, and affecting the adsorption rate. achieve the effect of improving the legibility of the drawing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pechini Synthesis
[0586]Although any metal salt or combination of metal salts can be combined and processed using the Pechini method to prepare metal oxide catalysts, this example uses Ca, Nd, and Sr salts to prepare a mixed metal oxide OCM catalyst. Equimolar aqueous solutions of strontium nitrate, neodymium nitrate, and calcium nitrate were prepared. Aliquots of each solution were mixed together to prepare a desired formulation of CaxNdySrz where x, y and z each independently represent mole fractions of total metal content in moles. Representative examples of formulations include, but are not limited to, Ca50Nd30Sr20, Ca52Nd45Sr05, Ca75Nd22Sr03, and the like. A solution of citric acid was added to the metal salt mixture so that the citric acid mole / metal mole ratio was 3:1. Ethylene glycol (or any polyfunctional alcohol, for example glycerol or polyvinyl alcohol) was then added to the citric acid / metal salt solution so that the ethylene glycol / citric acid mole ratio was 1:1. The so...
example 2
Polymer Templated Synthesis
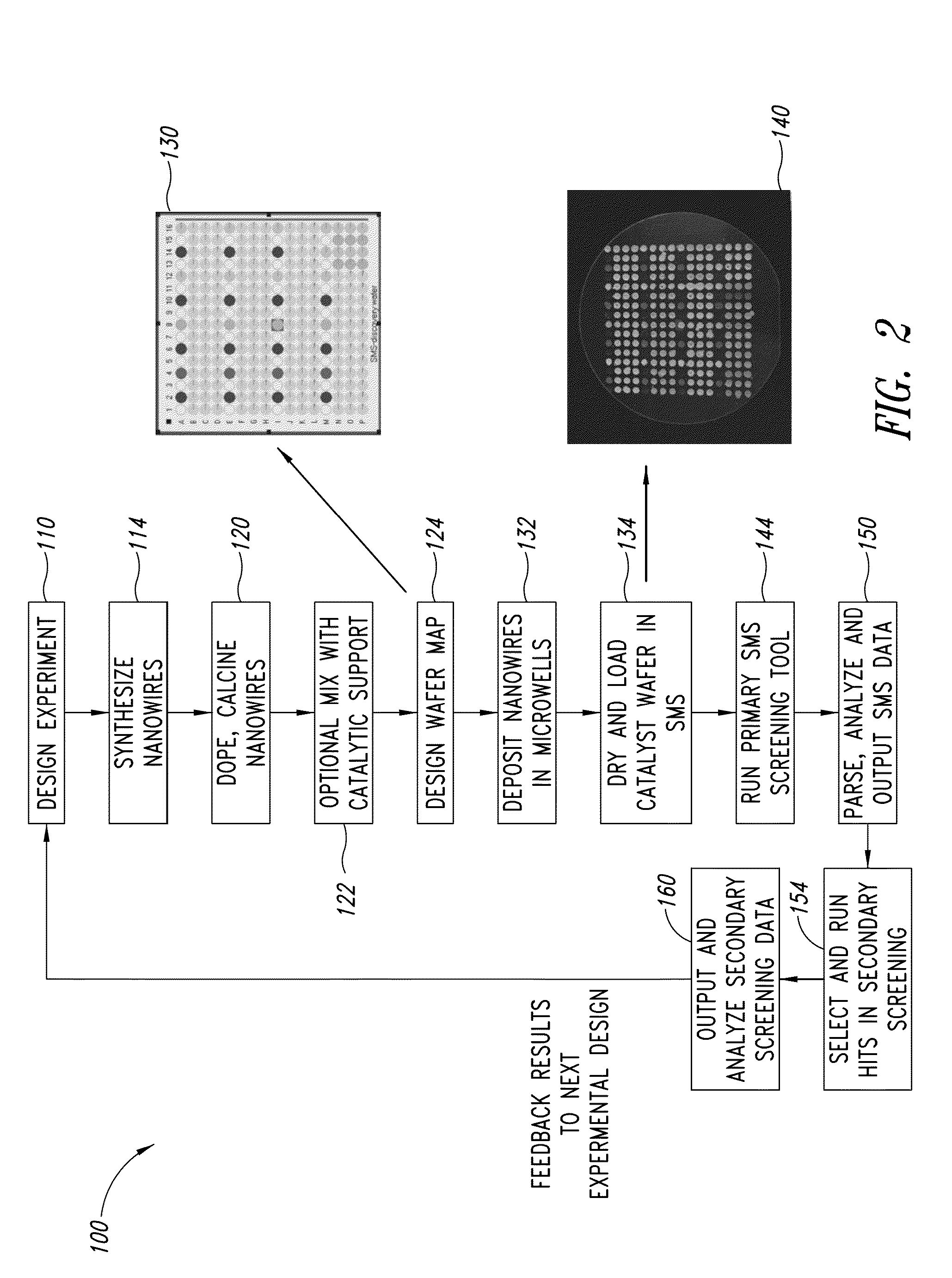
[0587]Dextran is a water-soluble polymer with a wide range of molecular weights and is a useful templating source. Briefly, a metal precursor and dextran are dissolved in water to produce a viscous solution. The solution is dried to make a metal organic composite and then calcined (oven or microwave) to remove the dextran template. Optionally, multiple metal precursors are dissolved in a dextran solution. Mixed metal oxide materials are readily prepared by dissolving different metal salts, in the desired ratio, in a viscous dextran solution. The solution is dried and calcined as described above to yield mixed metal oxide systems for OCM catalysis. Alternatively, freeze drying may be employed to dry the dextran / metal solution to prepare a more controllable porosity in the metal and mixed metal oxide materials.
[0588]Agarose is also used to prepare metal and mixed metal oxides for OCM catalysts. Agarose readily forms a gel that can be used as templating sourc...
example 3
Preparation Mg(OH)2 Nanowires
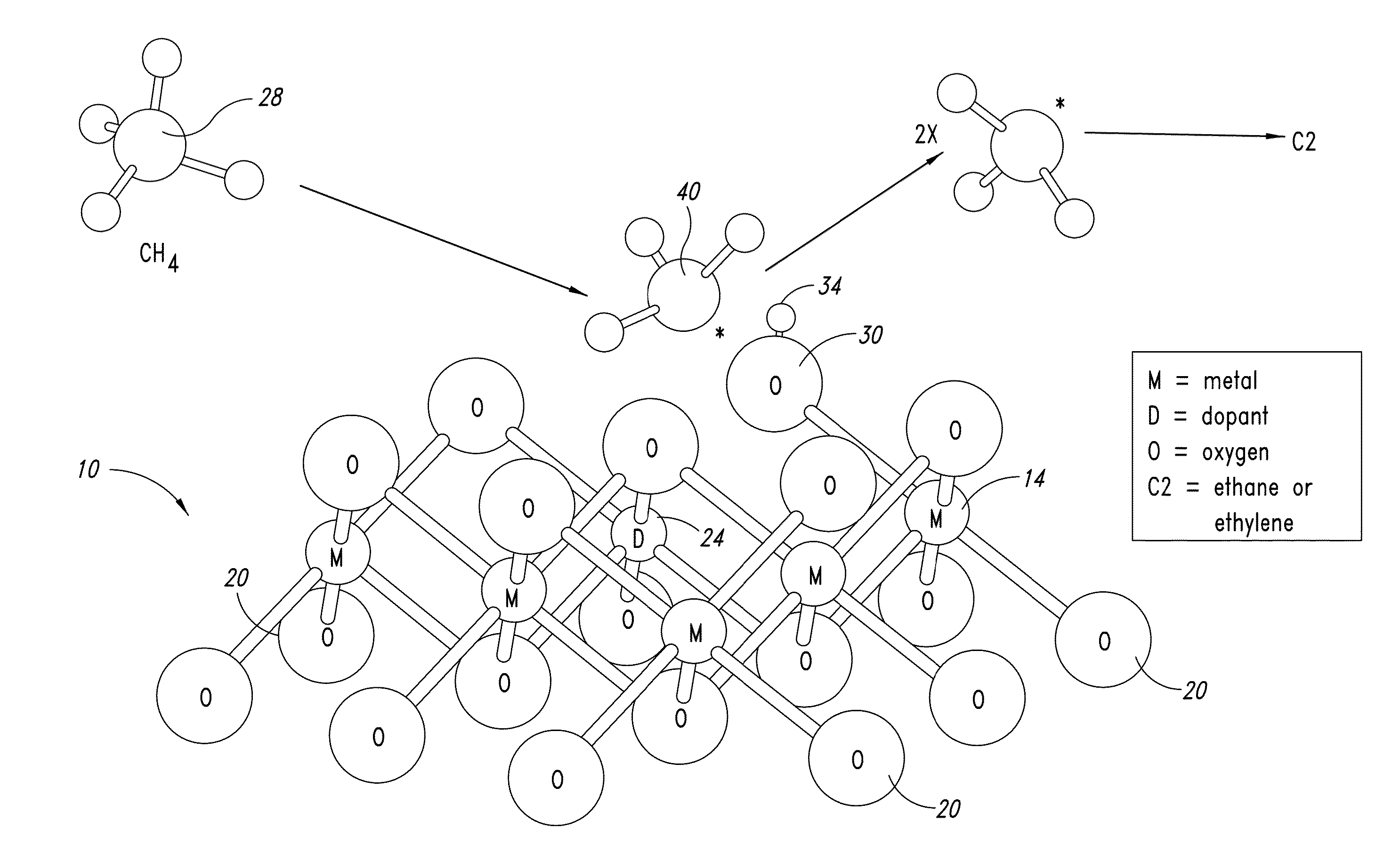
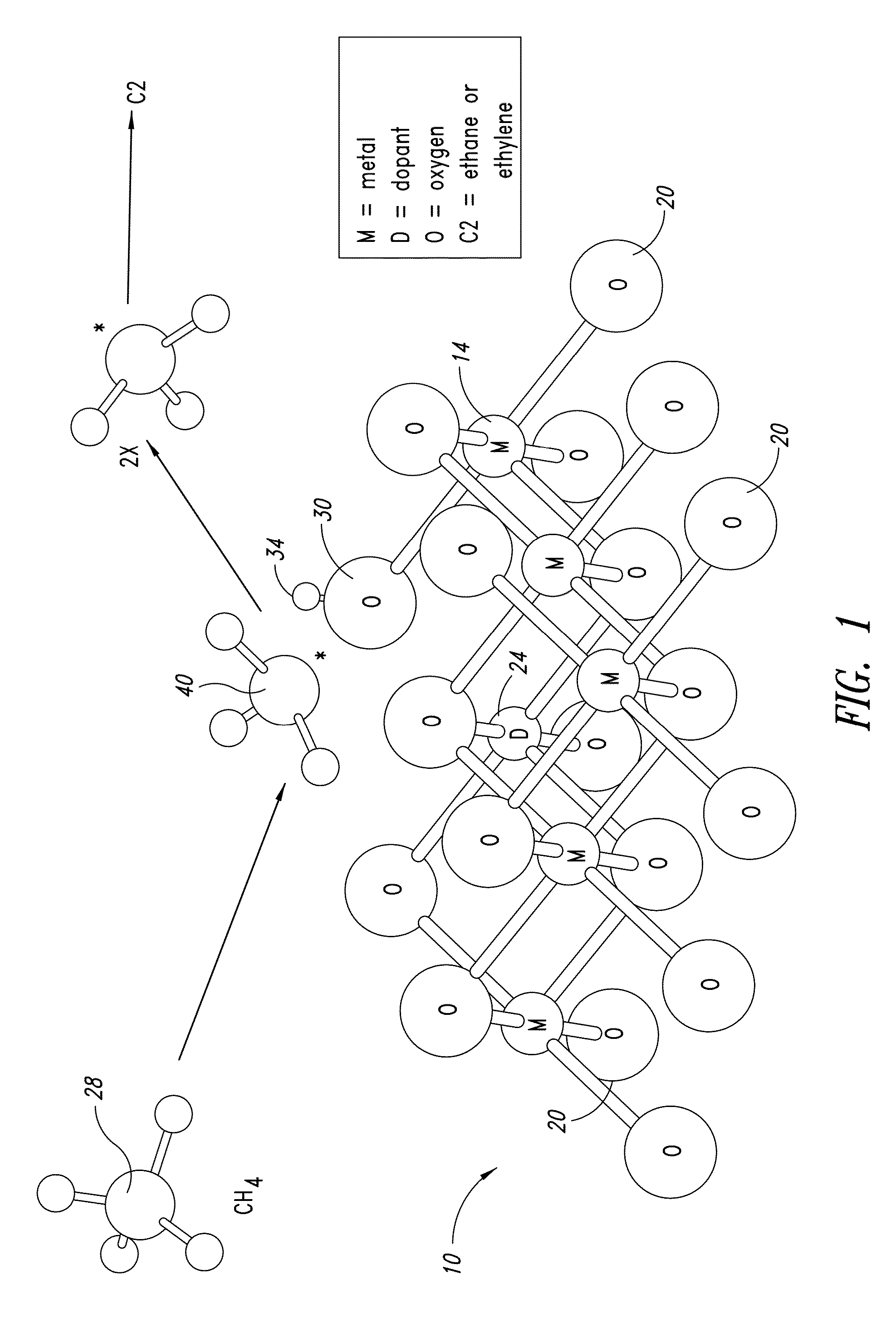
[0591]FIG. 11 shows a generic reaction scheme for preparing MgO nanowires (with dopant) via a polymer template. The reaction container can be anything from a small vial (for milliliter scale reactions) up to large bottles (for liter reaction scale reactions).
[0592]A magnesium solution and a base solution are added to the polymer solution in order to precipitate Mg(OH)2. The magnesium solution can be of any soluble magnesium salt, e.g. MgX2.6H2O (X═Cl, Br, I), Mg(NO3)2, MgSO4, magnesium acetate, etc. The range of the magnesium concentration in the reaction mixture is quite narrow, typically at 0.01M. The combination of the polymer concentration and the magnesium concentration (i.e. the ratio between the polymer and magnesium ions) can play a part in determining both the nanowires formation process window and their morphology.
[0593]The base can be any alkali metal hydroxide (e.g. LiOH, NaOH, KOH), soluble alkaline earth metal hydroxide (e.g. Sr(OH)2, Ba(OH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com