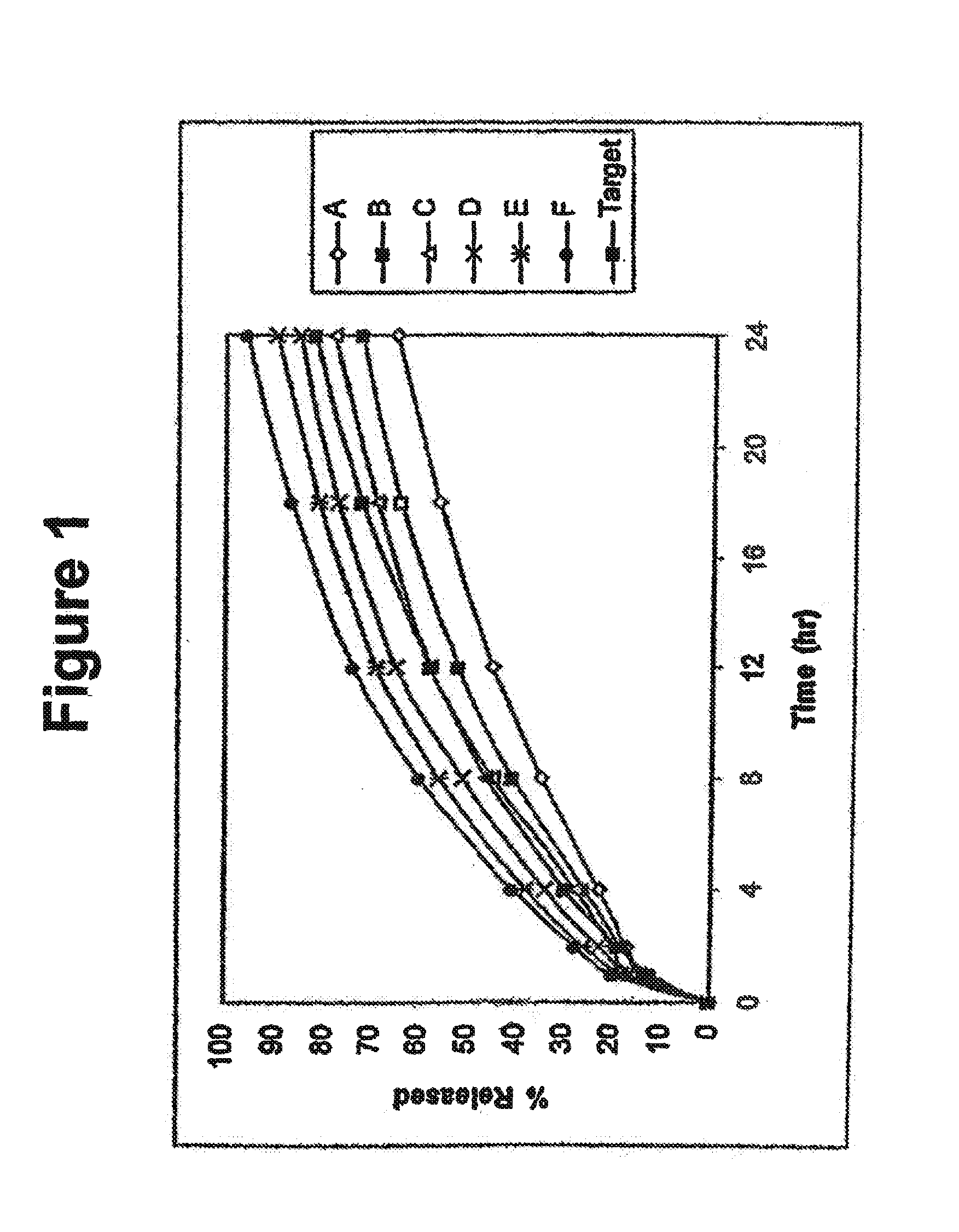
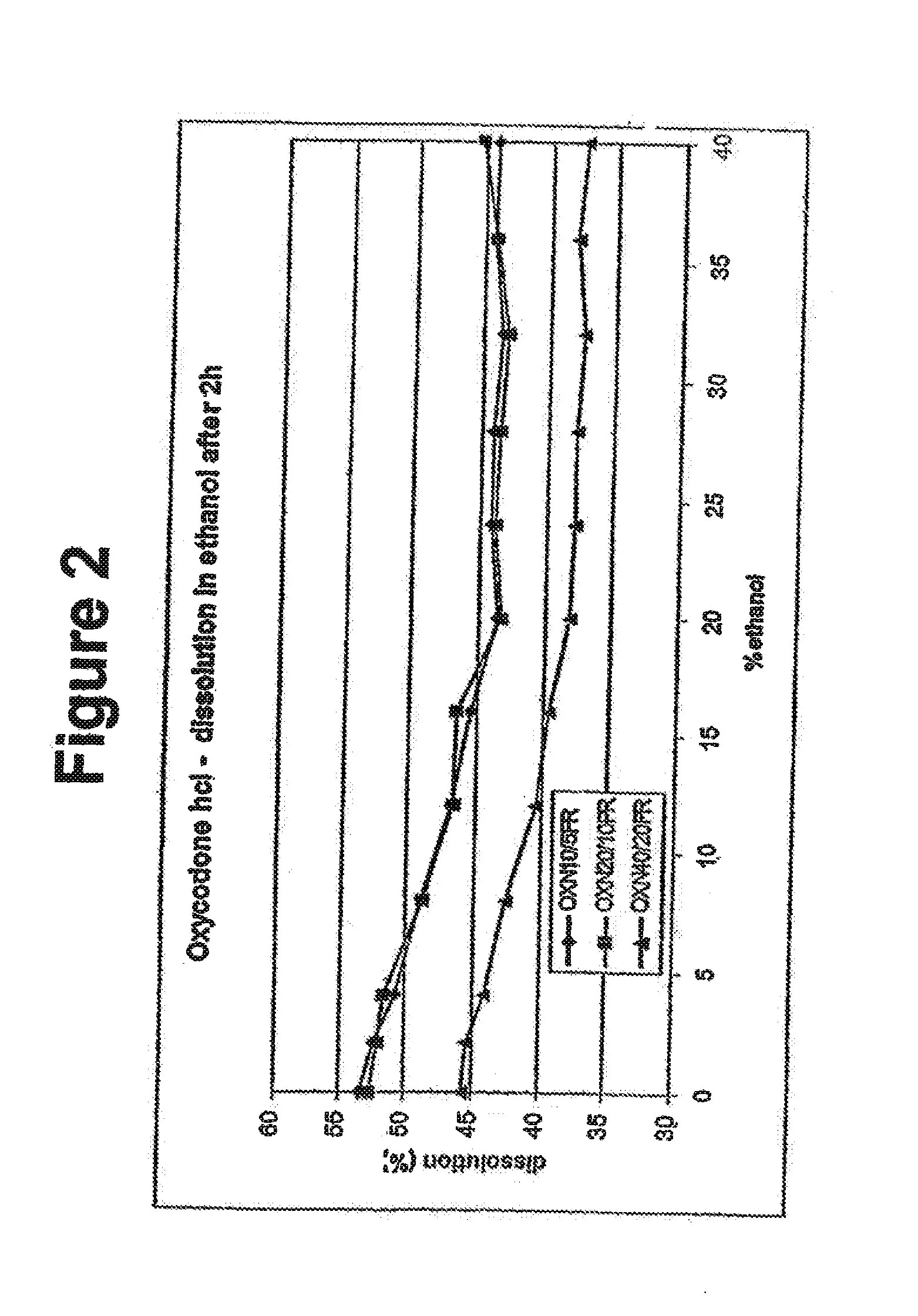
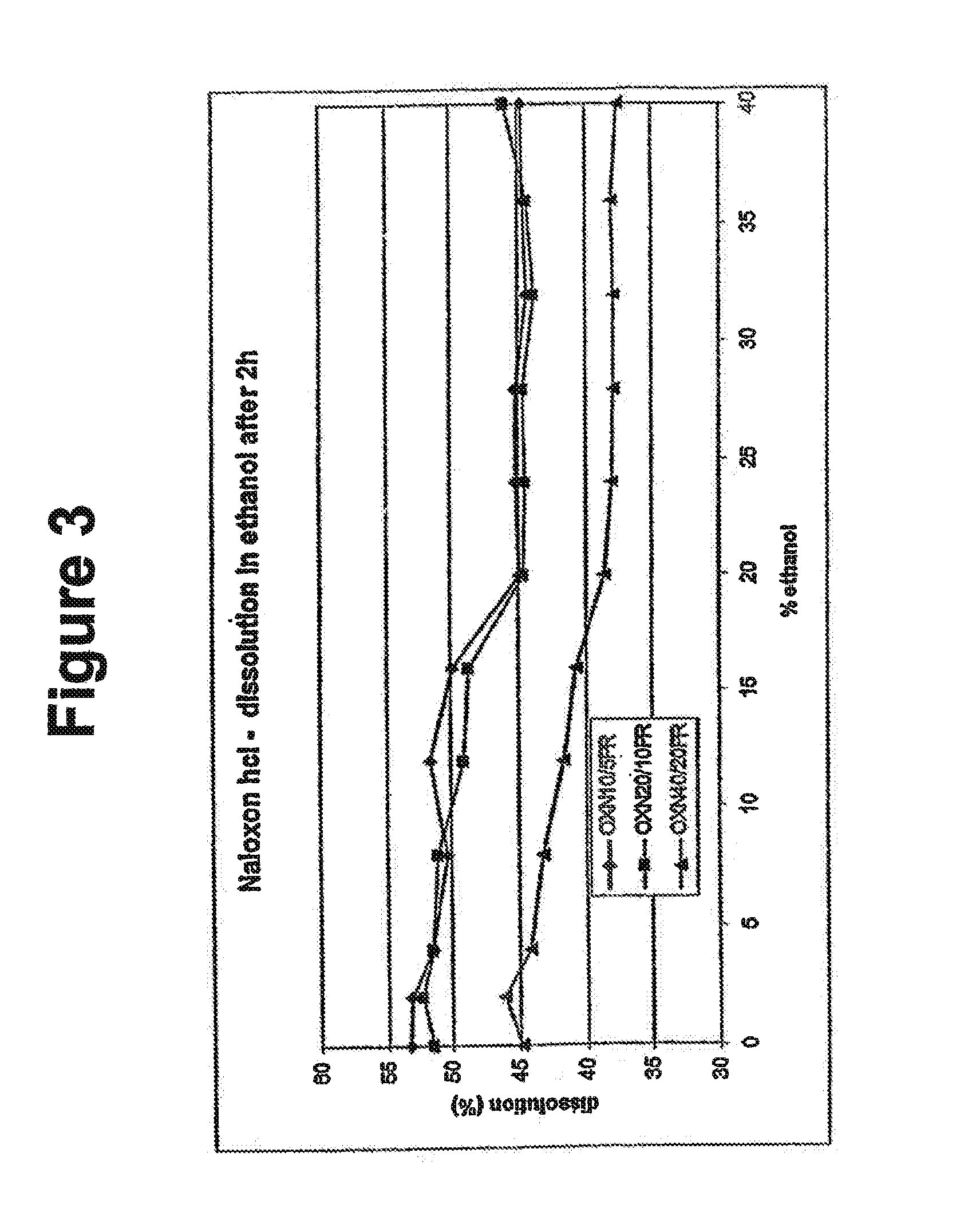
Alcohol resistant dosage forms
a technology of alcohol-resistant dosage forms and controlled release formulations, which is applied in the direction of drug compositions, biocide, heterocyclic compound active ingredients, etc., can solve the problems of rapid release and absorption of hydromorphone from the formulation, drug products are sometimes subject to abuse, and patients receiving doses that are more rapid than patients, so as to achieve the effect of resisting alcohol extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparative Example
[0125]Example 1 is the approved Palladone (sustained release hydromorphone hydrochloride) formulation and contains the following ingredients:
Hydromorphone HCl12.0 mgEudragit RSPO*76.5 mgEthylcellulose 4.5 mgStearyl alcohol27.0 mg*(poly(meth)acrylate with 5% trimethylammoniummethacrylate chloride)
[0126]The formulation was prepared by the following procedure:[0127]1. Mill the stearyl alcohol.[0128]2. Blend the Hydromorphone HCl, ethylcellulose, Eudragit RSPO and milled stearyl alcohol on a v-blender[0129]3. Extrude the blend from (1) using a ZSE-218 extruder fitted with counter-rotating screws, and a 1 mm die plate. Using a pelletizer, cut the strands to create cylindrical pellets approximately 1 mm long and 1 mm in diameter.
example 2.1
Example 2.1
[0130]The composition of Example 2.1 is summarized below.
Amt / unitAmt / batchIngredient (Trade Name)(mg)(g)Hydromorphone HCl12.0221.1*Ethycellulose (Ethocel Std. Premium 7)61.01,118.3Glyceryl palmitostearate (Precirol ATO 5)27.0495.0Hydroxypropyl Cellulose (Klucel EF)20.0366.7Total120.02201.1*Weigh corrected for water and impurities (99.5% based on Certificate of Analysis)
[0131]The processing conditions at the time of sampling are summarized below.
Extruder: Leistritz ZSE 27
Screw Configuration: Counter-rotation
[0132]
Heating Zone123-67-89-1011-12Temperature (° C.)1540125125125124-125
Condition #1
Torque (%): 25
[0133]Melt Pressure (psi): 480
Feed rate (kg / hour): 2.9
Screw speed (rpm): 90
Die Plate Hole diameter (mm): 1.0 (8-hole die plate)
Condition #2
Torque (%): 25
[0134]Melt Pressure (psi): 520
[0135]Feed rate (kg / hour): 4.2
Screw speed (rpm): 90
Die Plate Hole diameter (mm): 1.0 (8-hole die plate)
[0136]The processing steps for manufacturing the Hydromorphone HCl 12 mg melt extruded mu...
example 2.2
[0143]Example 2.2 compares the impact of various concentrations of ethanol in simulated gastric fluid (500 ml in Example 1; 900 ml in Example 2.1) using a USP Apparatus I (basket) apparatus at 100 rpm at 37 degrees C.° on the dissolution of the current Palladone formulation and the formulation of Example 2.1 containing the same concentration of hydromorphone (19% w / w). The current Palladone formulation contains an ammonio methacrylate copolymer as the primary release-rate controlling excipient whereas the formulation of Example 2.1 contains ethylcellulose. The results are summarized below.
BathVessel60 minutesExample 11 (SGF Media)11%2 (10% EtOH in SGF)39%3 (15% EtOH in SGF)64%4 (20% EtOH in SGF)88%5 (40% EtOH in SGF)97%Example 2.11 (SGF Media, pH = 1.27)13%2 (11% EtOH in SGF)15%3 (20% EtOH in SGF)19%4 (25% EtOH in SGF)23%5 (35% EtOH in SGF)33%6 (SIF Media, pH = 7.43)13%
[0144]The data show that the formulation of Example 2.1 is more resistant to increases in the drug release in the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com