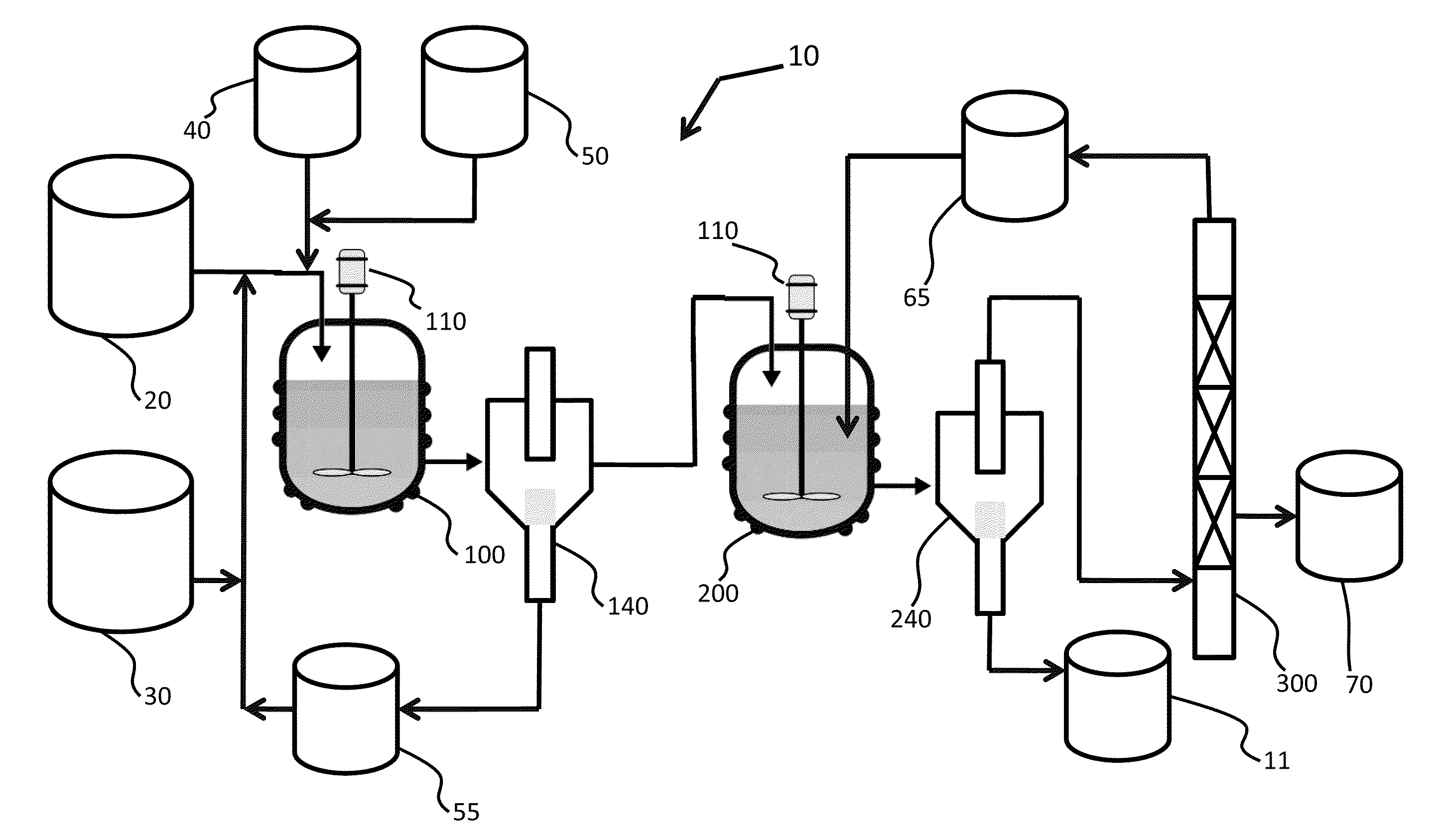
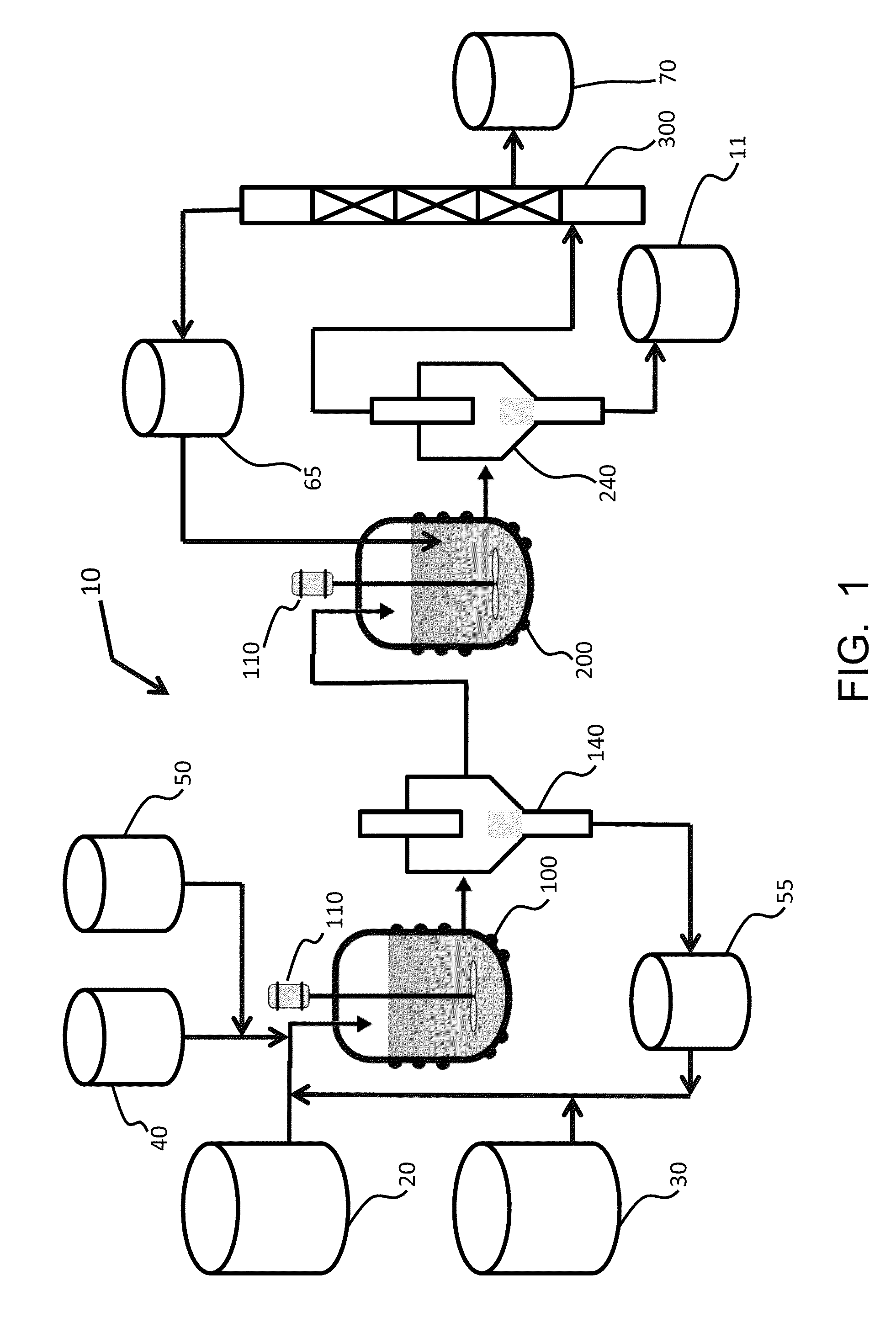
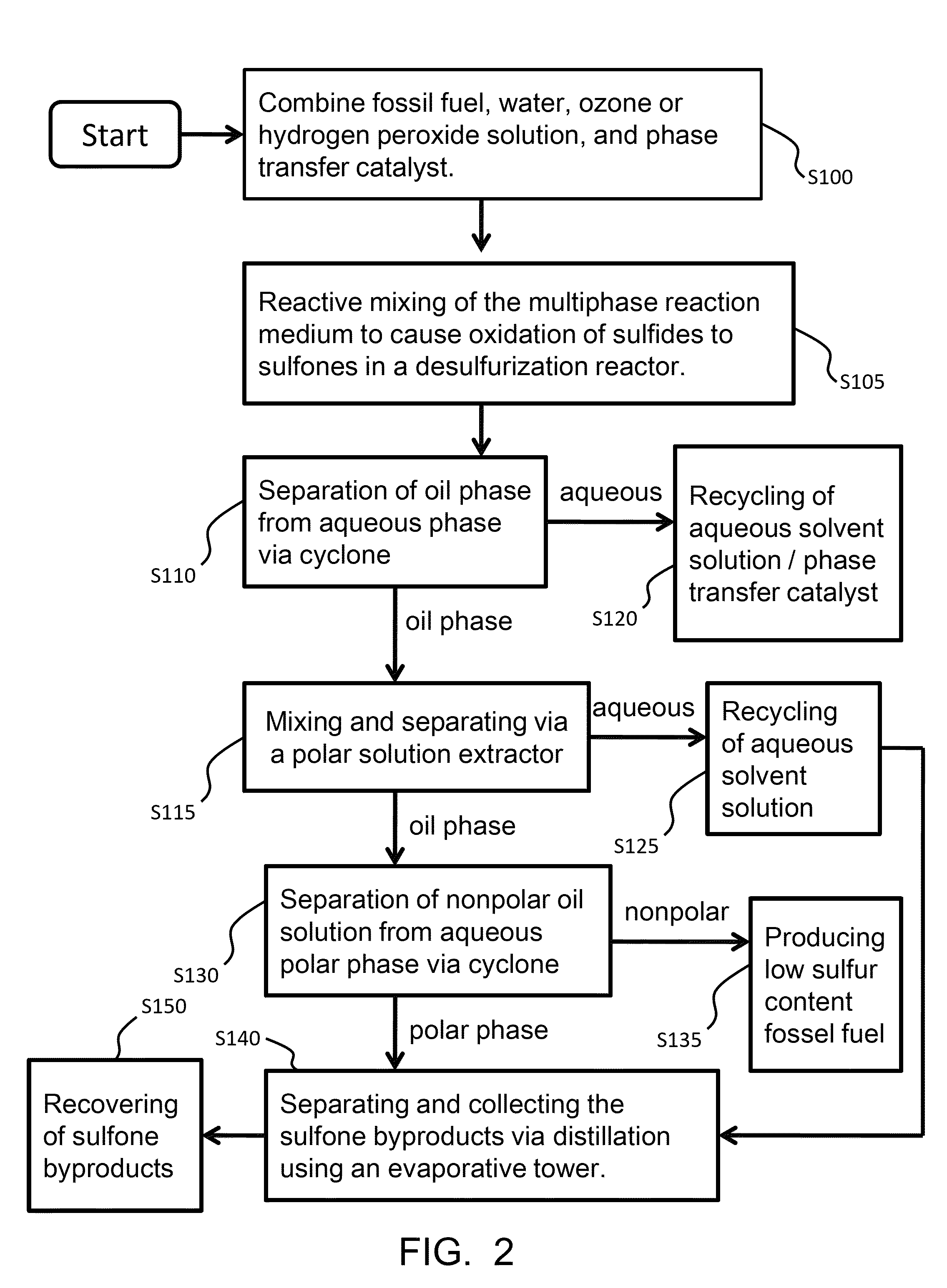
Mixing-assisted oxidative desulfurization of diesel fuel using quaternary ammonium salt and portable unit thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016]Quaternary ammonium salts are compounds comprised of a positively charged nitrogen atom having four substituents, paired with a negatively charged counterion.
[0017]The term “hydroperoxide” is used herein to denote a compound of the molecular structure in which R represents either a hydrogen atom or an organic or inorganic group. Examples of hydroperoxides in which R is an organic group are water-soluble hydroperoxides such as methyl hydroperoxide, ethyl hydroperoxide, isopropyl hydroperoxide, n-butyl hydroperoxide, sec-butyl hydroperoxide, tert-butyl hydroperoxide, 2-methoxy-2-propyl hydroperoxide, tert-amyl hydroperoxide, and cyclohexyl hydroperoxide. Examples of hydroperoxides in which R is an inorganic group are peroxonitrous acid, peroxophosphoric acid, and peroxosulfuric acid. Preferred hydroperoxides are hydrogen peroxide (in which R is a hydrogen atom) and tertiary-alkyl peroxides, notably tert-butyl peroxide.
[0018]The aqueous fluid that is combined with the fossil fuel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com