Pharmaceutical formulations useful in the treatment of insomnia
a technology of pharmaceutical formulations and insomnia, applied in the direction of drug compositions, biocide, heterocyclic compound active ingredients, etc., can solve the problems of function of normal people, affecting the quality of life of people, adverse personal, medical or psychiatric effects, and inability to sleep normally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Sublingual Tablets
[0080]Sublingual tablets comprising 5 mg and 10 mg of zolpidem hemitartrate were prepared as follows.
[0081]Zolpidem hemitartrate (Boehringer Ingelheim, Germany) was firstly ground for 20 minutes in a ball mill.
[0082]The active ingredient was then accurately weighed out, along with the other excipients (see below), in appropriate proportions that would enable the production of tablets with the absolute amounts of various ingredients mentioned below.
[0083]Pre-weighed quantities of zolpidem hemitartrate and mannitol (Parteck M200; Merck, Germany) were then mixed in a Turbula mixer for 96 hours. Then, pre-weighed quantities of silicified microcrystalline cellulose (ProSolv®; JRS Pharma, Germany), sodium carboxymethylcellulose (Croscarmellose Sodium NF; Ac-Di-Sol®; FMC Corp., USA), Neohesperidin DC (Exquim, Spain) and peppermint powder (Firmenich, Germany) were added and mixing was continued for 30 minutes. Finally, a pre-weighed quantity of magnesium ste...
example 2
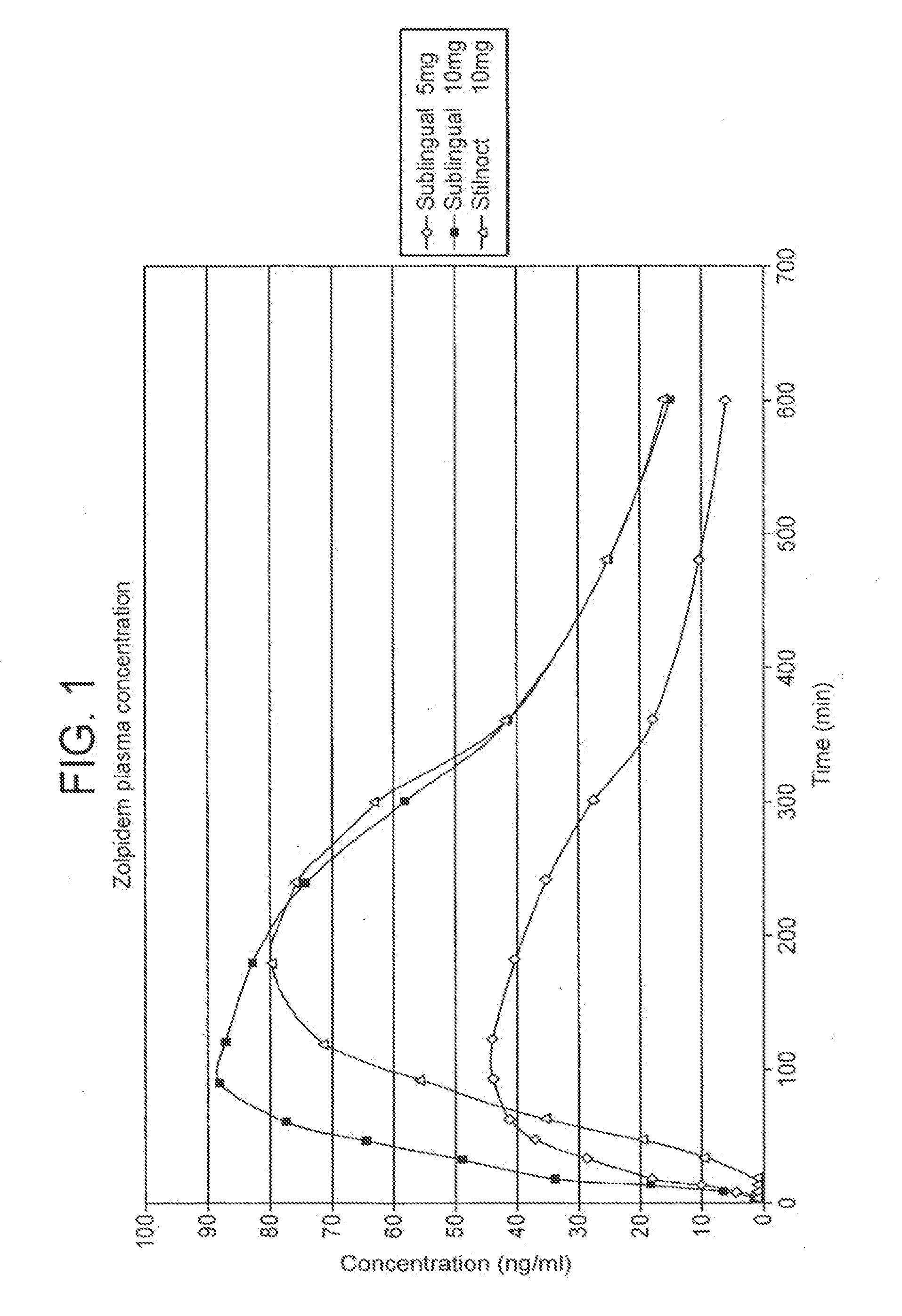
[0087]An open randomized three-period crossover single-centre study was devised to evaluate the pharmacokinetic profile of the sublingual zolpidem 5 mg and 10 mg tablets prepared by way of Example 1 above, as compared to a peroral zolpidem formulation (Stilnoct® 10 mg; Sanofi-Synthélabo, France).
[0088]The trial was a pharmacokinetic study in healthy male and female volunteers to test for dose proportionality as between the two sublingual tablet formulations. The pharmacokinetic profiles were evaluated, focussing on bioavailability and time and rate of absorption. The study also included a subjective assessment of efficacy, i.e. the subjects' perceived degree of sedation.
[0089]18 healthy subjects aged between 18 and 40 were used in this study. Signed informed consents were obtained in all cases.
[0090]Each of the three formulations were given to each of the 18 volunteers, in a random order, at three visits to the study centre (hereinafter “Visits 1, 2 and 3”). Visits 1 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com