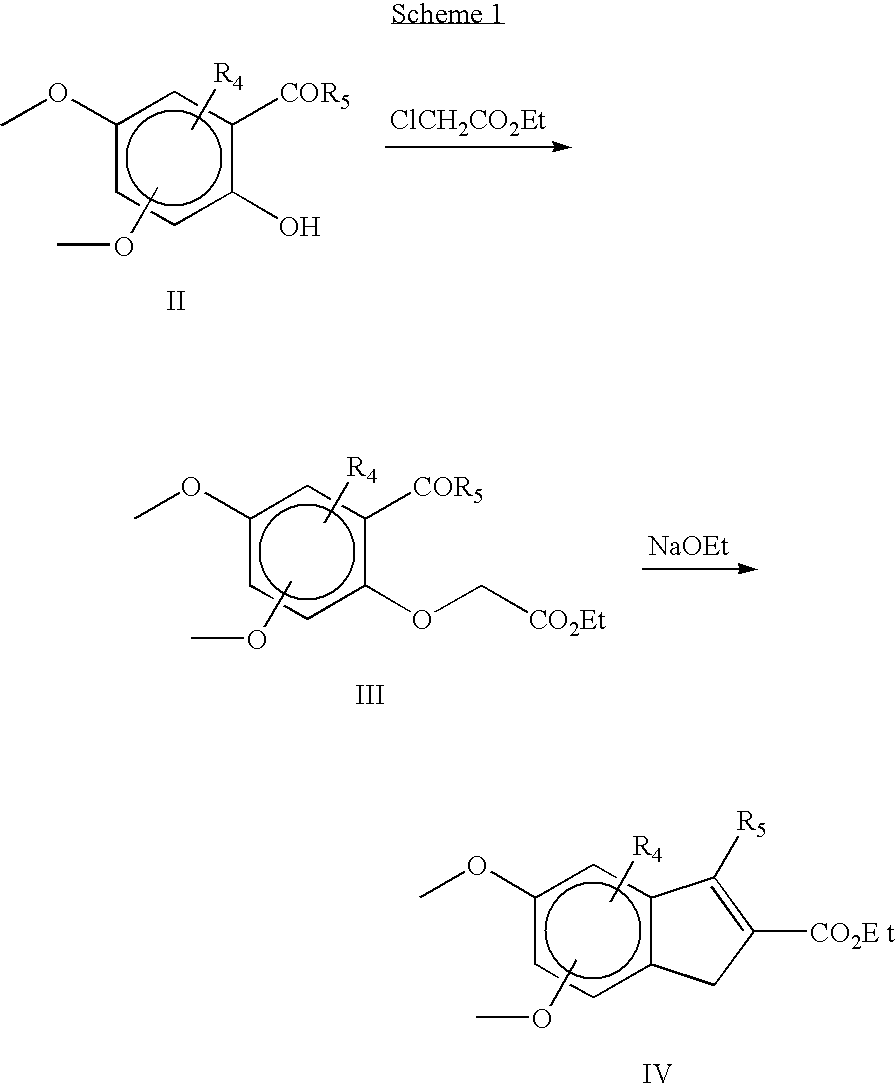
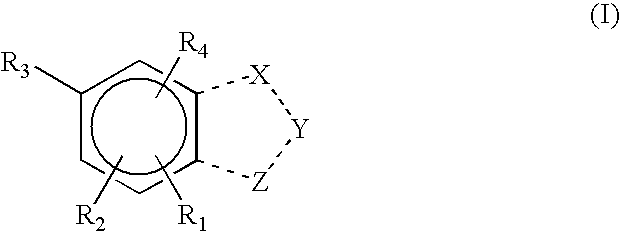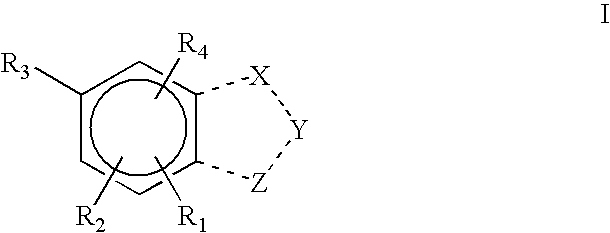Pharmaceutical compounds
a technology of benzofused and heterocyclic compounds, applied in the field of pharmaceutical compounds, can solve the problems of limited oral bioavailability and short duration of action, and achieve the effects of prolonging the duration of action, enhancing primary pharmacological properties, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-(4-Chloro-phenyl)-5,6-dihydroxy-4-nitro-2,3-dihydro-isoindol-1-one
2-(4-Chloro-phenyl)-5,6-dimethoxy-2,3-dihydro-isoindol-1-one
[0070] A solution of 2-bromomethyl-4,5-dimethoxy-benzoic acid methyl ester (2.9 g), 4-chloroaniline (1.28 g) and triethylamine (1.4 ml) was refluxed in toluene for six hours. The reaction mixture was stirred in an ice bath, filtered and washed with 1 M hydrochloric acid and water.
[0071] Yield: 0.74 g
[0072]1H NMR (DMSO-d6): δ=3.85 (s, 3H, CH3O), 3.88 (s, 3H, CH3O), 4.89 (s, 2H, CH2), 7.23 (s, 1H, ArH), 7.24 (s, 1H, ArH), 7.48 (d, 2H, J=8.9 Hz), 7.91 (d, 2H, J=8.9 Hz).
2-(4-Chloro-phenyl)-5,6-dihydroxy-2,3-dihydro-isoindol-1-one
[0073] 2-(4-Chloro-phenyl)-5,6-dimethoxy-2,3-dihydro-isoindol-1-one (0.74 g) was demethylated with 4 eq of boron tribromide as described in Example 8.
[0074] Yield: 0.79 g (raw material used as such in the next step)
[0075]1H NMR (DMSO-d6): δ=4.80 (s, 2H, CH2), 6.77 (s, 1H, ArH), 6.82 (s, 1H, ArH), 7.48 (d, 2H, J=9.1 Hz), 7.91 (d,...
example 2
5,6-Dihydroxy-7-nitro-3H-isobenzofuran-1-one
5,6-Dihydroxy-7-nitro-3H-isobenzofuran-1-one
[0088] To a solution of 5,6-dihydroxy-3H-isobenzofuran-1-one (0.4 g) in sulfuric acid at −30° C. was added 5 M nitric acid in sulfuric acid (0.55 ml). The reaction mixture was let to warm up to room temperature and then poured into ice water. The product was filtered and recrystallized from acetic acid.
[0089] Yield: 0.2 g
[0090]1H NMR (DMSO-d6): δ=5.26 (s, 2H, CH2), 7.14 (s, 1H, ArH), 10.6 (b, 1H, OH), 11.75 (b, 1H, OH).
example 3
7-Nitro-2-pyridin-4-yl-benzothiazole-5,6-diol, methane sulfonate
5,6-Dimethoxy-2-pyridin-4-yl-benzothiazole
[0091] A solution of 3,4-dimethoxyaniline (6 g) and sulfur (5 g) were refluxed in 4-picolin (15 ml) for five hours. The cool reaction mixture was poured into methanol, kept over ice bath for minutes and filtered. The product was washed with methanol and carbon disulfide.
[0092] Yield: 6.14 g
[0093]1H NMR (DMSO-d6): δ=3.88 (s, 3H, CH3O), 3.89 (s, 3H, CH3O), 7.67 (s, 1H, ArH), 7.76 (s, 1H, ArH), 7.94 (d, 2H, J=6.4 Hz), 8.75 (d, 2H, J=6.4 Hz).
5,6-Dimethoxy-4-nitro-2-pyridin-4-yl-benzothiazole
[0094] To a solution of 5,6-dimethoxy-2-pyridin-4-yl-benzothiazole (1.1 g) in sulfuric acid (10 ml) was added potassium nitrate (0.5 g). After 60 minutes in room temperature the mixture was poured into ice water and filtered. Recrystallization from acetone yielded the pure product.
[0095] Yield: 0.8 g
[0096]1H NMR (DMSO-d6): δ=4.05 (s, 3H, CH3O), 4.07 (s, 3H, CH3O), 8.07 (d, 2H, J=6 Hz), 8....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com