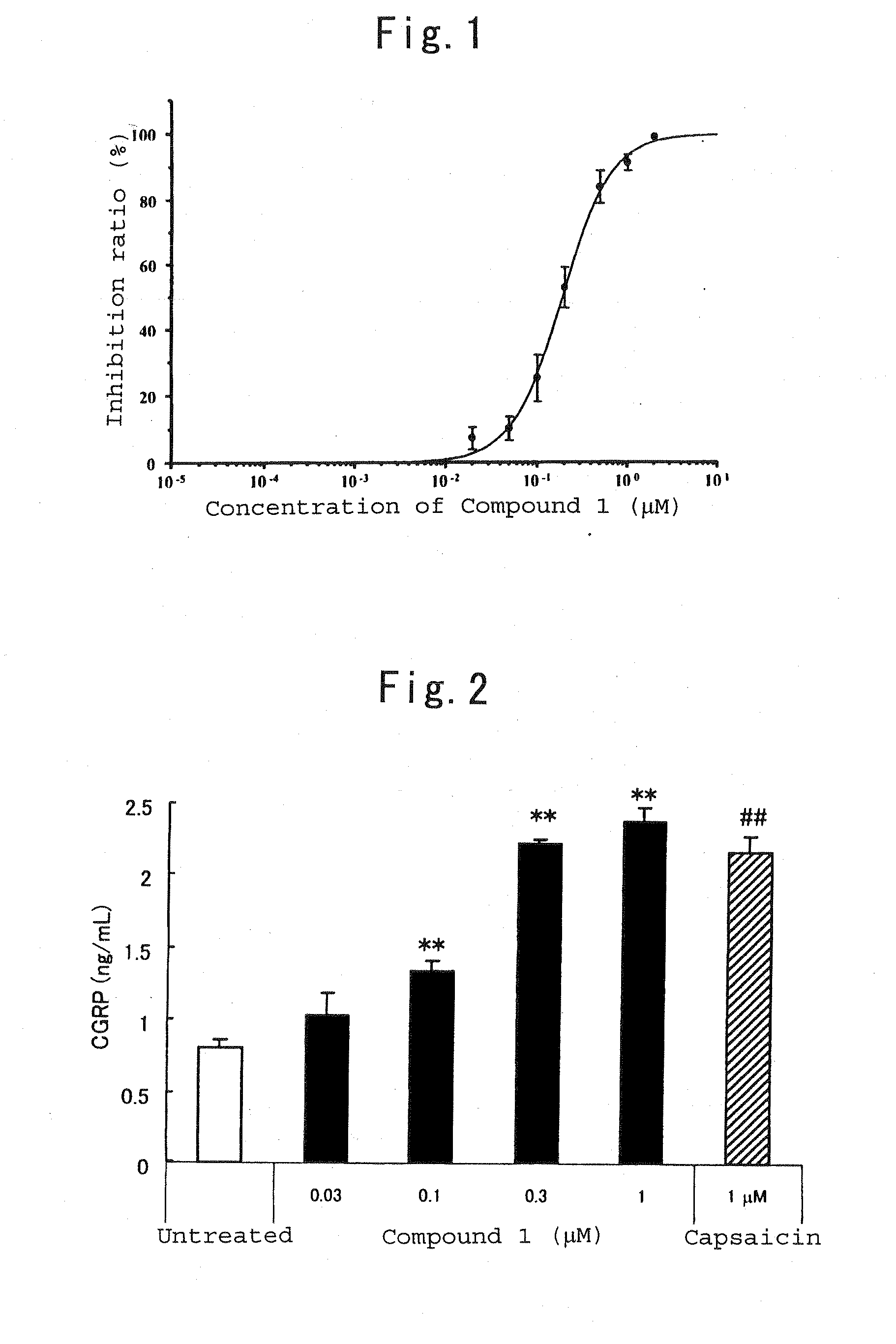
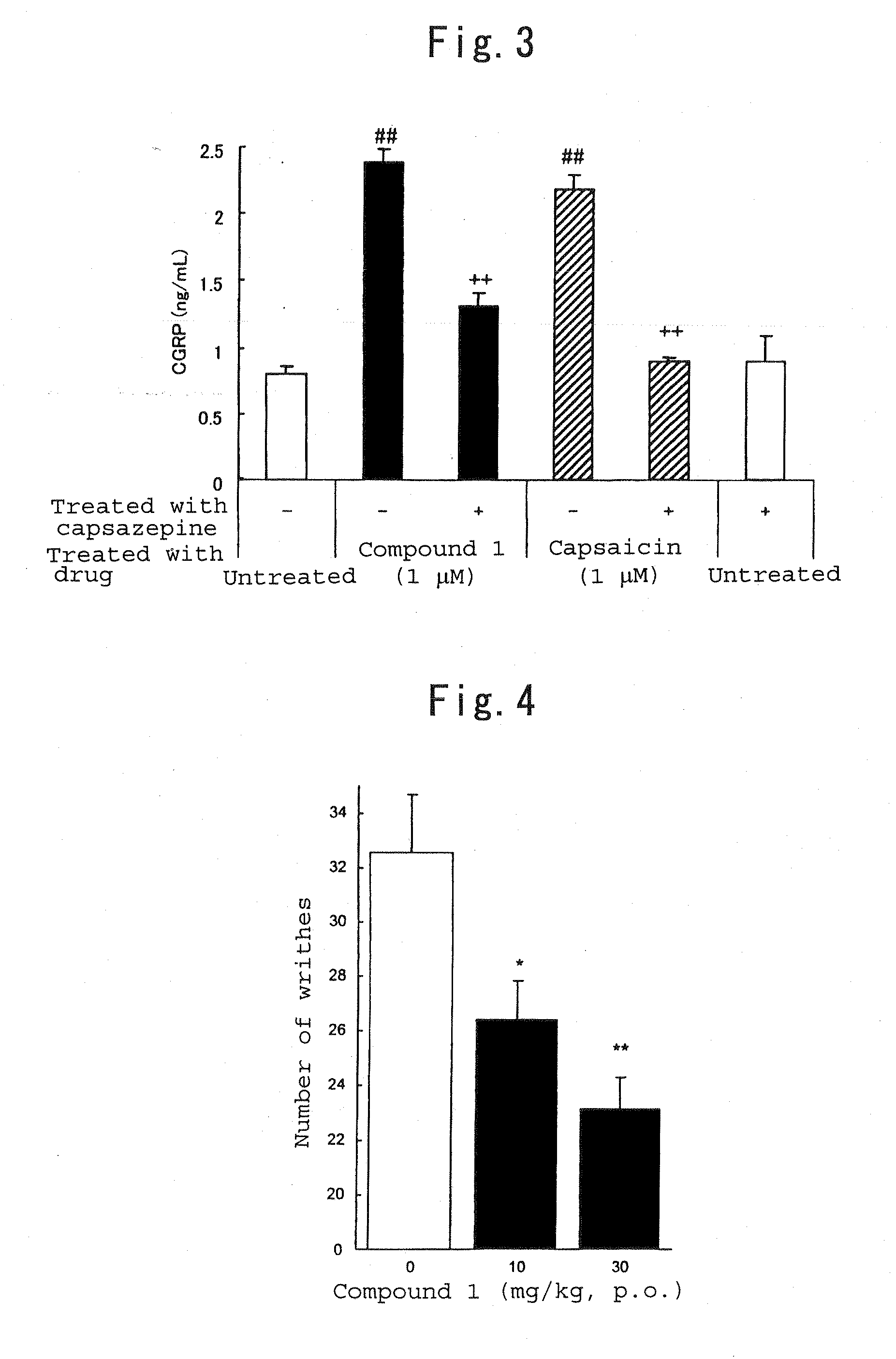
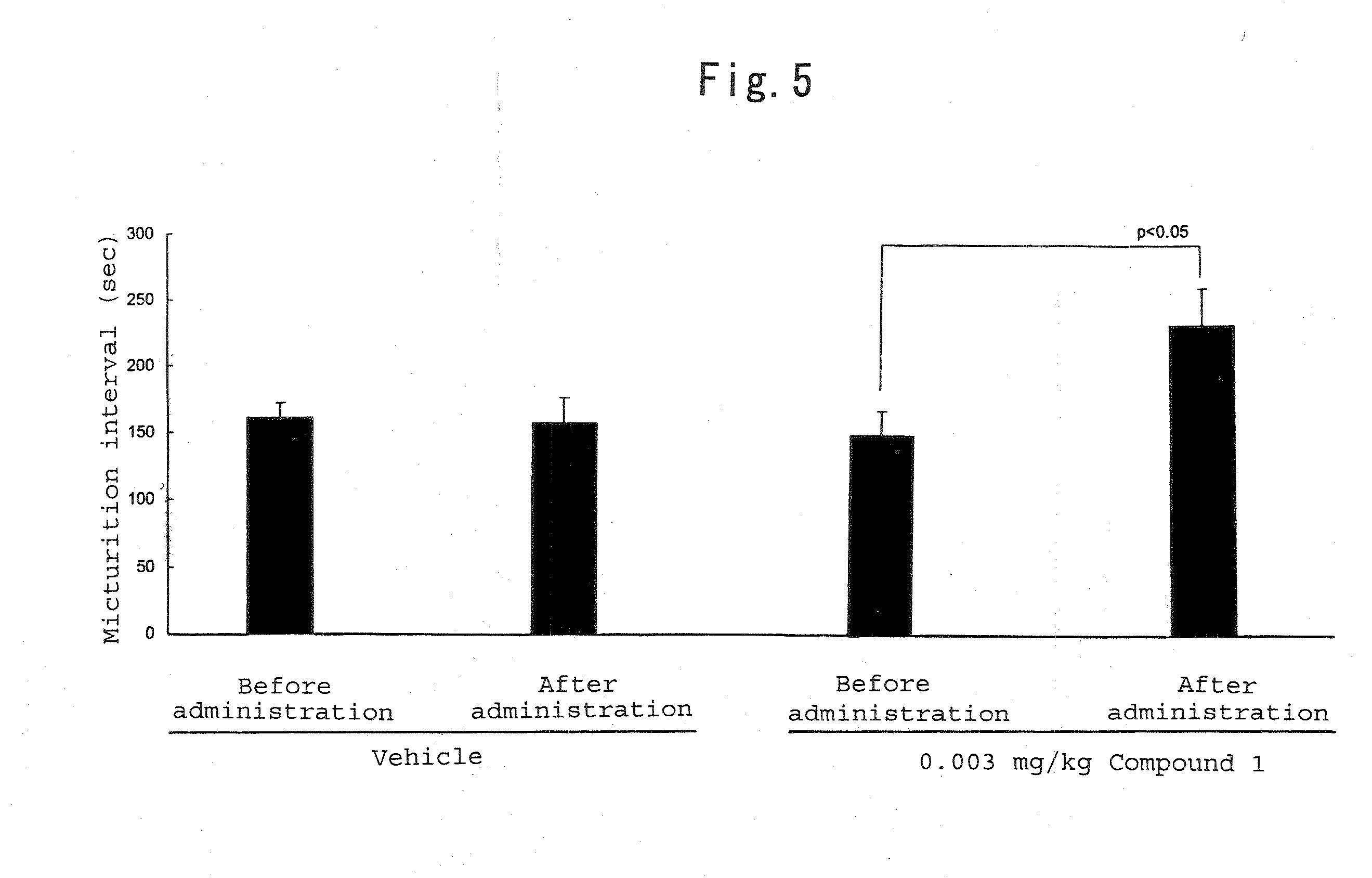
Therapeutic agent for trpv1-mediated disease
a technology of trpv1 and therapeutic agent, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, metabolic disorders, etc., can solve the problems of ulcer recurrence, medication compliance sometimes decreases, and patients cannot achieve satisfactory therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example
[0078]General preparation examples of the present compound as an oral preparation and an injection are shown below.
1) Tablet
[0079]
Formulation 1 (in 100 mg)Present compound1 mgLactose66.4 mg Corn starch20 mg Carxboxymethylcellulose calcium6 mgHydroxypropylcellulose4 mgMagnesium stearate0.6 mg
[0080]A tablet of the above-mentioned formulation is coated using 2 mg of a coating agent (for example, a conventional coating agent such as hydroxypropylmethylcellulose, macrogol, or a silicone resin), whereby an objective coated tablet can be obtained (the same shall apply to a tablet of a formulation below). In addition, a desired tablet can be obtained by appropriately changing the amounts of the present compound and the additives.
2) Capsule
[0081]
Formulation 1 (in 150 mg)Present compound 5 mgLactose145 mg
[0082]A desired capsule can be obtained by appropriately changing the mixing ratio of the present compound to lactose.
3) Injection
[0083]
Formulation 1 (in 10 ml)Present compound10 to 100mgS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com