Controlled release compositions of agents that reduce circulating levels of platelets and methods therefor
a technology of circulating platelets and compositions, which is applied in the field of controlled release compositions of agents that reduce circulating platelets and methods therefor, can solve the problems of discontinuing the drug as a therapeutic, increasing the risk of thrombosis, so as to reduce the number of circulating platelets, and reduce the circulating platelet count
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0087]A. Definitions
[0088]B. The Role of Platelets and Health
[0089]C. Platelet-related conditions and diseases[0090]1. Thrombotic events[0091]2. Vaso-occlusive events[0092]3. Vascular disease[0093]4. Myeloproliferative disorders[0094]a. Essential thrombocythemia (ET)[0095]b. Polycythemia vera (PV)[0096]c. Idiopathic myelofibrosis (IM)[0097]5. Other conditions
[0098]D. Anagrelide[0099]1. Chemistry[0100]2. Metabolites[0101]3. Derivatives and Analogs of Anagrelide[0102]4. Pharmacokinetic properties[0103]5. Mechanism of action
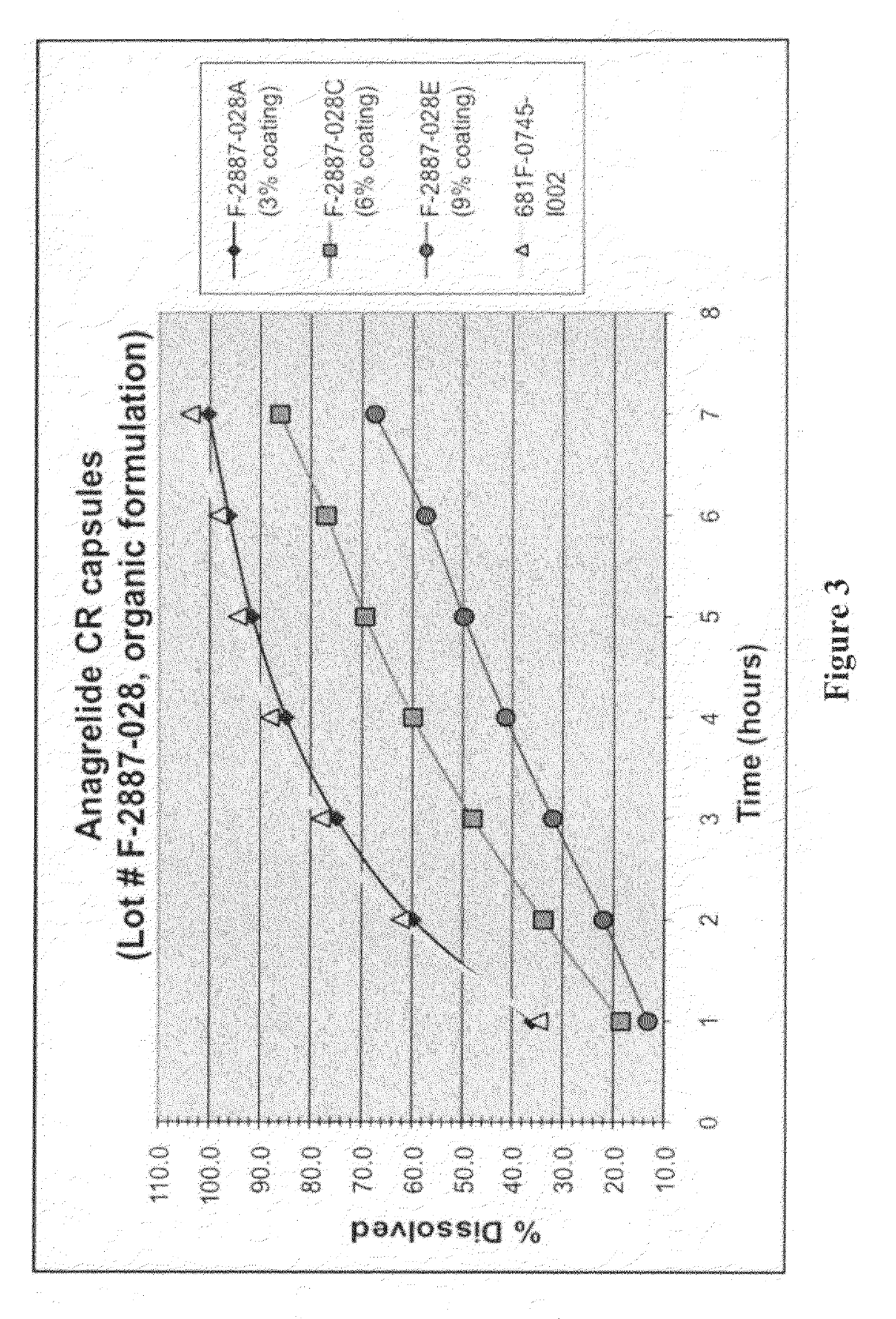
[0104]E. Compositions[0105]1. Form[0106]a. Cores[0107]b. Platelet number reducing agent[0108]c. Microparticles[0109]d. Coatings[0110]i. Optional preparatory coat[0111]ii. Substrate layer[0112]iii. Optional seal coat layer[0113]iv. Controlled release component[0114]v. Optional Finishing coat[0115]2. Pharmaceutical delivery forms[0116]a. Compositions for oral administration[0117]b. Compositions for other routes of administration
[0118]F. Methods of making coated partic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| half life | aaaaa | aaaaa |
| half life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com