Method of Treating Chronic Kidney Disease
a technology of chronic kidney disease and ferric organic compounds, which is applied in the field of pharmaceutical grade ferric organic compounds to treat chronic kidney disease, can solve the problems of reducing the damage of the nephron, affecting the survival and affecting the treatment of patients with esrd. the effect of preventing, preventing, and maintaining the progression of chronic kidney diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Method for Synthesis of a Pharmaceutical-Grade Ferric Organic Compound
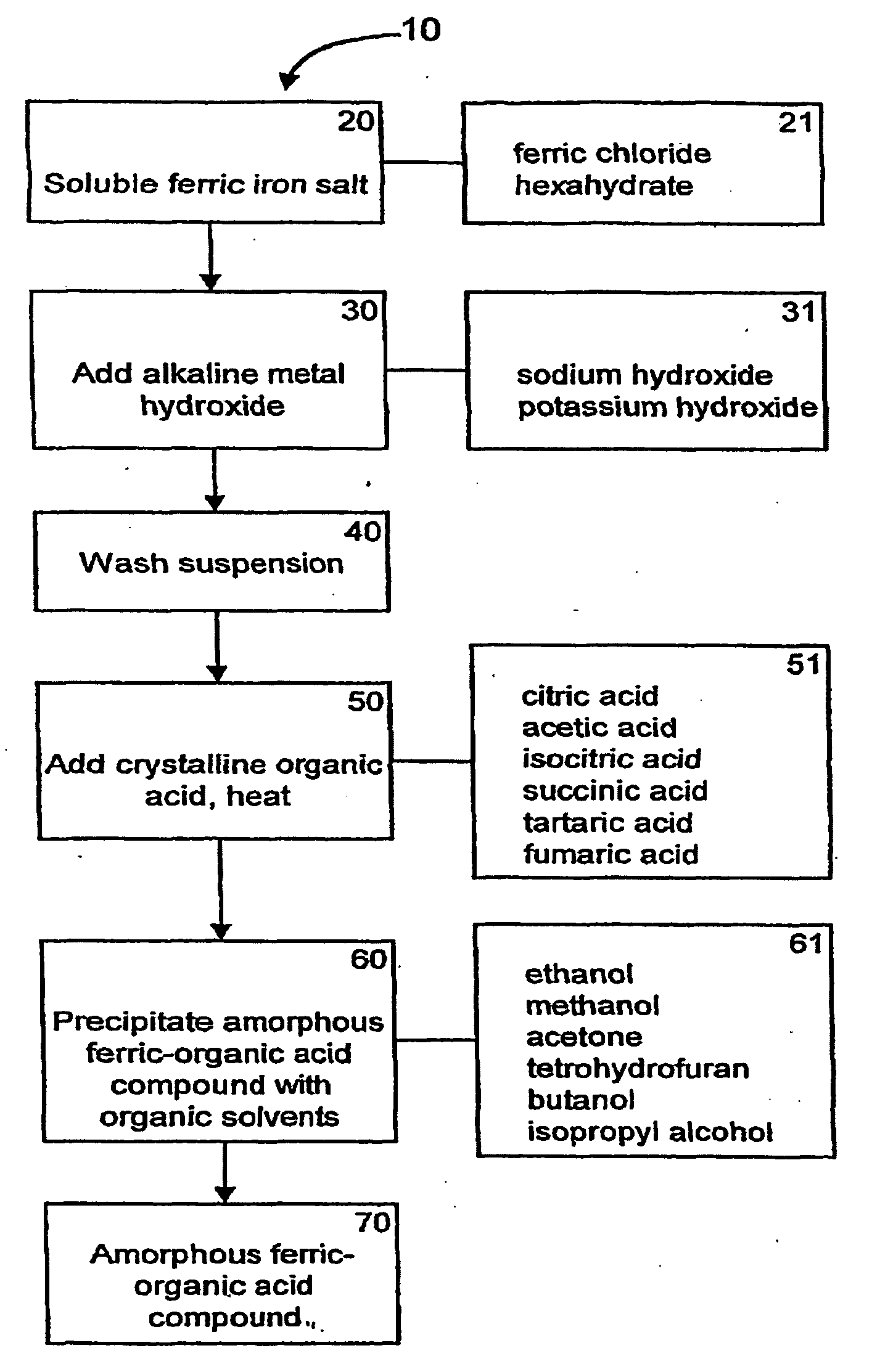
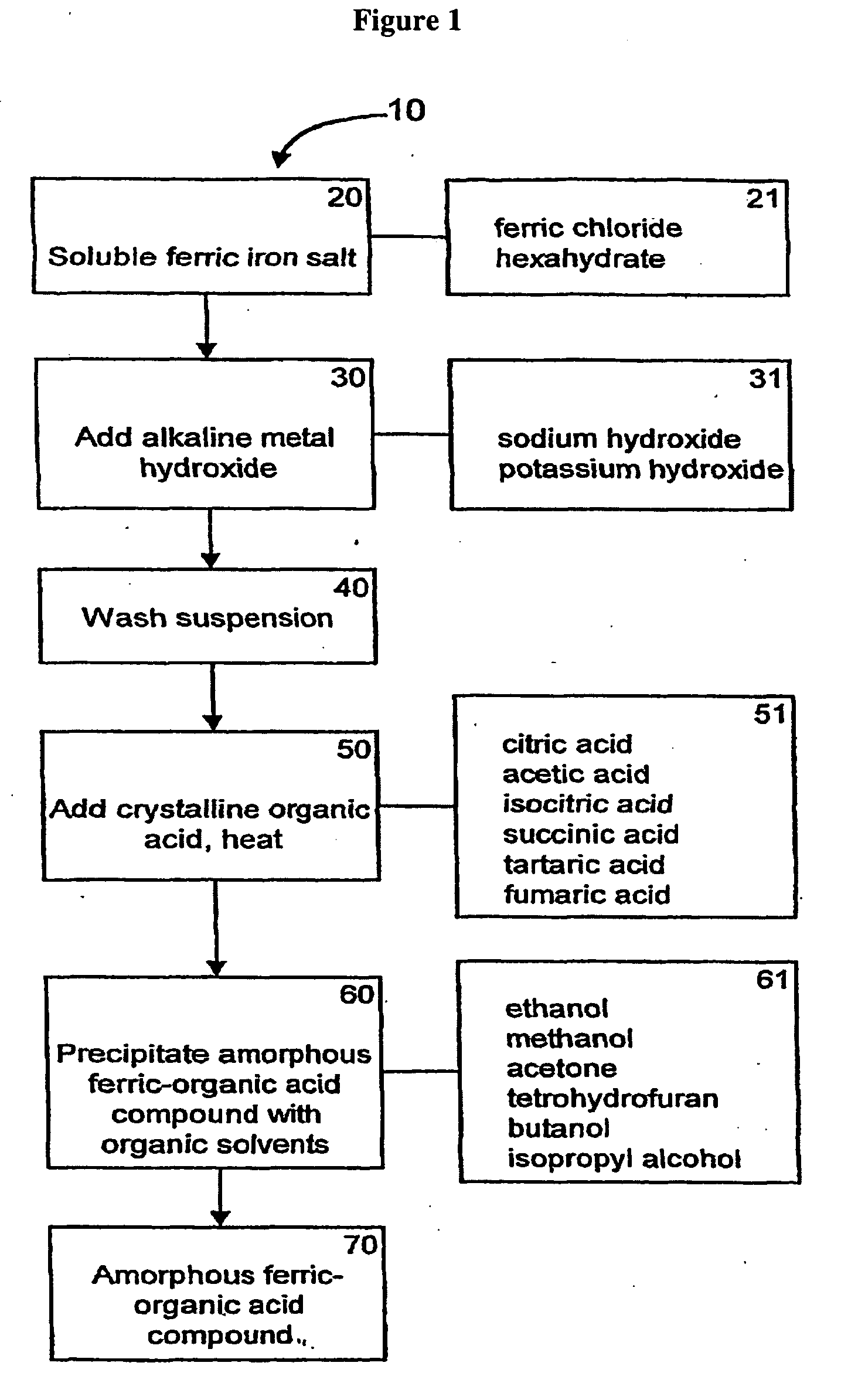
[0073]General methods for the synthesis of ferric organic compounds have been disclosed in PCT / US2006 / 032585, and U.S. provisional application No. 60 / 763,253, which are incorporated by reference into this application. Representative ferric organic compounds include, but are not limited to, ferric citrate.
[0074]Referring to FIG. 1, the flowchart 10 is a general process for synthesizing a form of ferric organic compound or ferric citrate compound which can be used in the present invention. The starting materials, as indicated in box 20, comprise soluble ferric iron salts. The soluble ferric iron salts can comprise ferric chloride hexahydrate (FeCl36 H2O), as indicated in box 21, or any other suitable soluble ferric iron salt. Next, an alkaline metal hydroxide (box 30) is added at a specific rate and temperature to the soluble ferric iron salt. The addition of the alkaline metal hydroxide at a specific rate, ...
example 2
Solubility Profile of Ferric Organic Compounds According to the Invention
[0079]The ferric organic compounds produced according to the methods described above are more soluble than commercially available ferric organic compounds, over a wider range of pH levels. This increase in solubility of the ferric organic compounds of the present invention is believed to be a result of the unique significantly large active surface area of the ferric organic compounds of the present invention. For example, at pH 8.0, the intrinsic dissolution rate of ferric citrate of the present invention is 3.32 times greater than the commercially available ferric citrate. See Table 1.
[0080]The intrinsic dissolution rates of commercially available ferric citrate were compared with the ferric citrate of the present invention. The intrinsic dissolution rate is defined as the dissolution rate of pure substances under the condition of constant surface area. The dissolution rate and bioavailability of a drug substa...
example 3
Methods o Using and Testing the Pharmaceutical-Grade Ferric Citrate in Patients
Handling and Forms of Test Compositions
[0083]Ferric citrate is supplied in 500 mg capsules, whereas the placebo will be provided in identical-looking capsules (indistinguishable from those containing the active drug); the placebo capsules will contain sorbitol and colorant to match the powder color of the active capsules. The placebo capsule shells will be identical to the active capsule shells.
Storage
[0084]All study drug supplies must be stored under secure conditions and are not to be used after their expiration date, which is imprinted on the study drug container. The study drugs should be kept under controlled conditions (15 to 30° C.; 59 to 86° F.) in a tightly closed container, protected from light.
Dosage
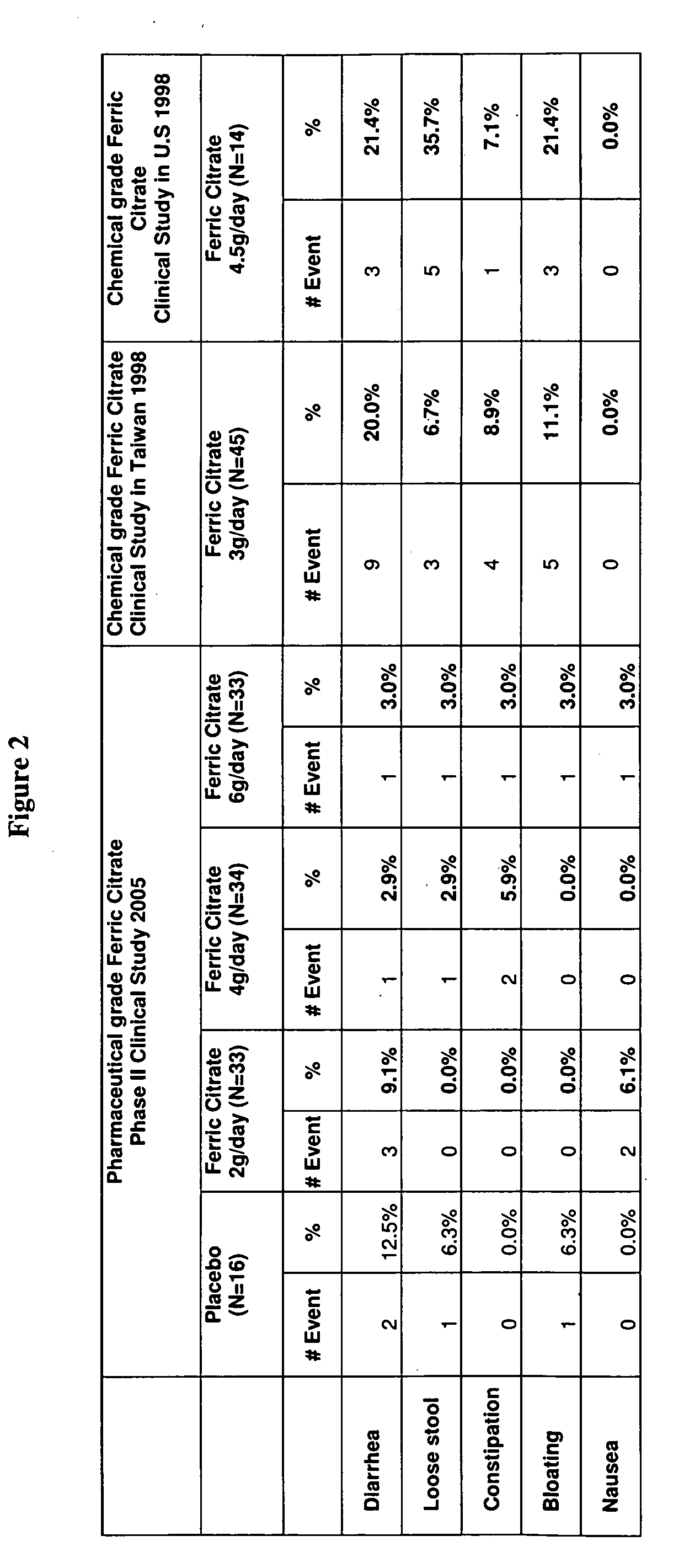
[0085]A recent pilot study compared ferric citrate (3 g daily) to calcium carbonate (3 g daily) for reducing serum P04 in patients with End Stage Renal Disease (ESRD). This dose of ferric citrate wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com