Process for Denitration of Exhaust Gas
a technology of denitration and exhaust gas, which is applied in the direction of separation processes, arsenic compounds, silicon compounds, etc., can solve the problems of increasing treatment costs, difficult application of non-catalytic denitration, and significant obstacles in the process of removing harmful substances, so as to improve the reactivity of nitrogen oxides, reduce the reductive decomposition of nitrogen oxides in the denitration step, and achieve the effect of efficient formation of amine radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
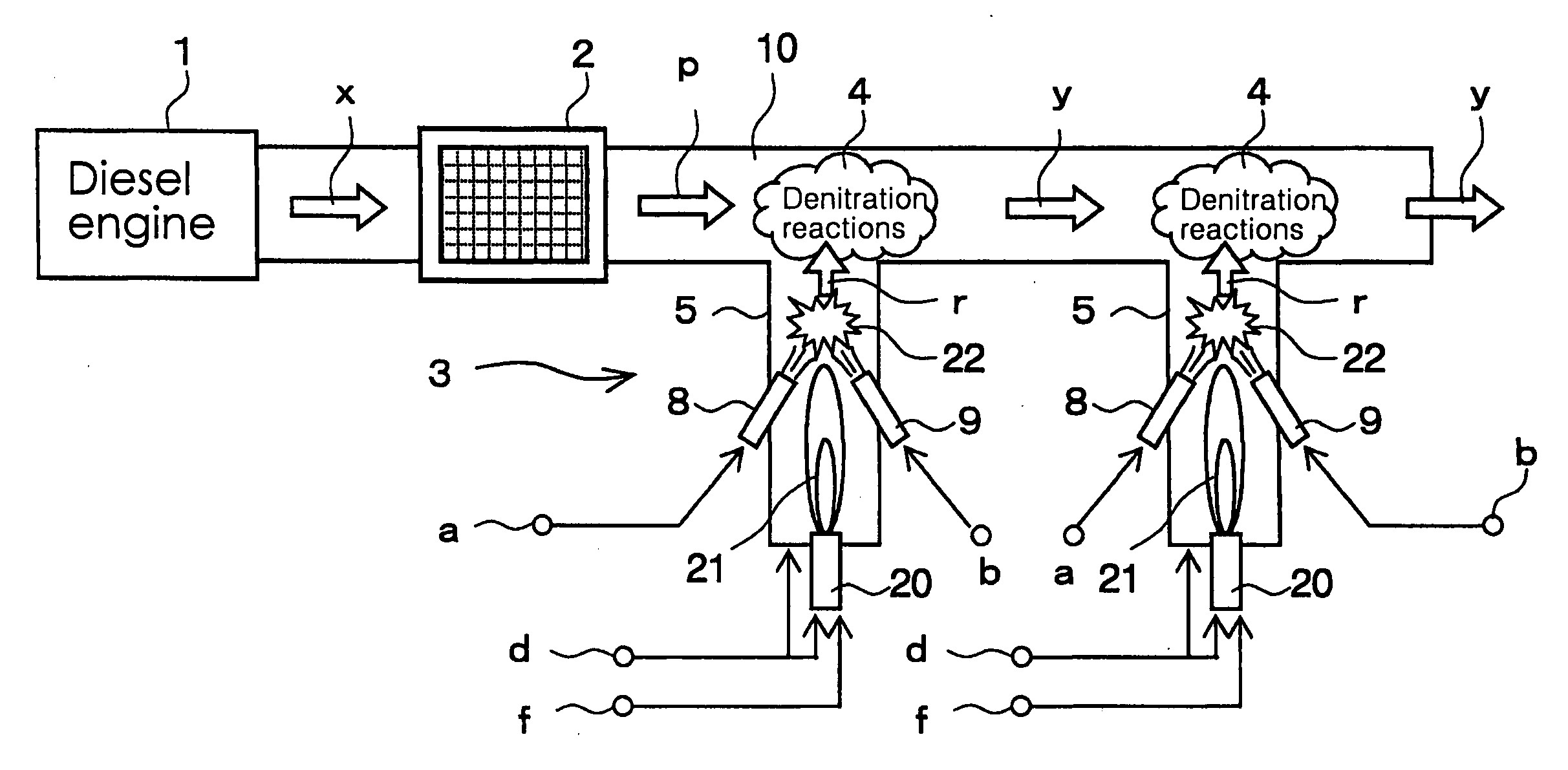
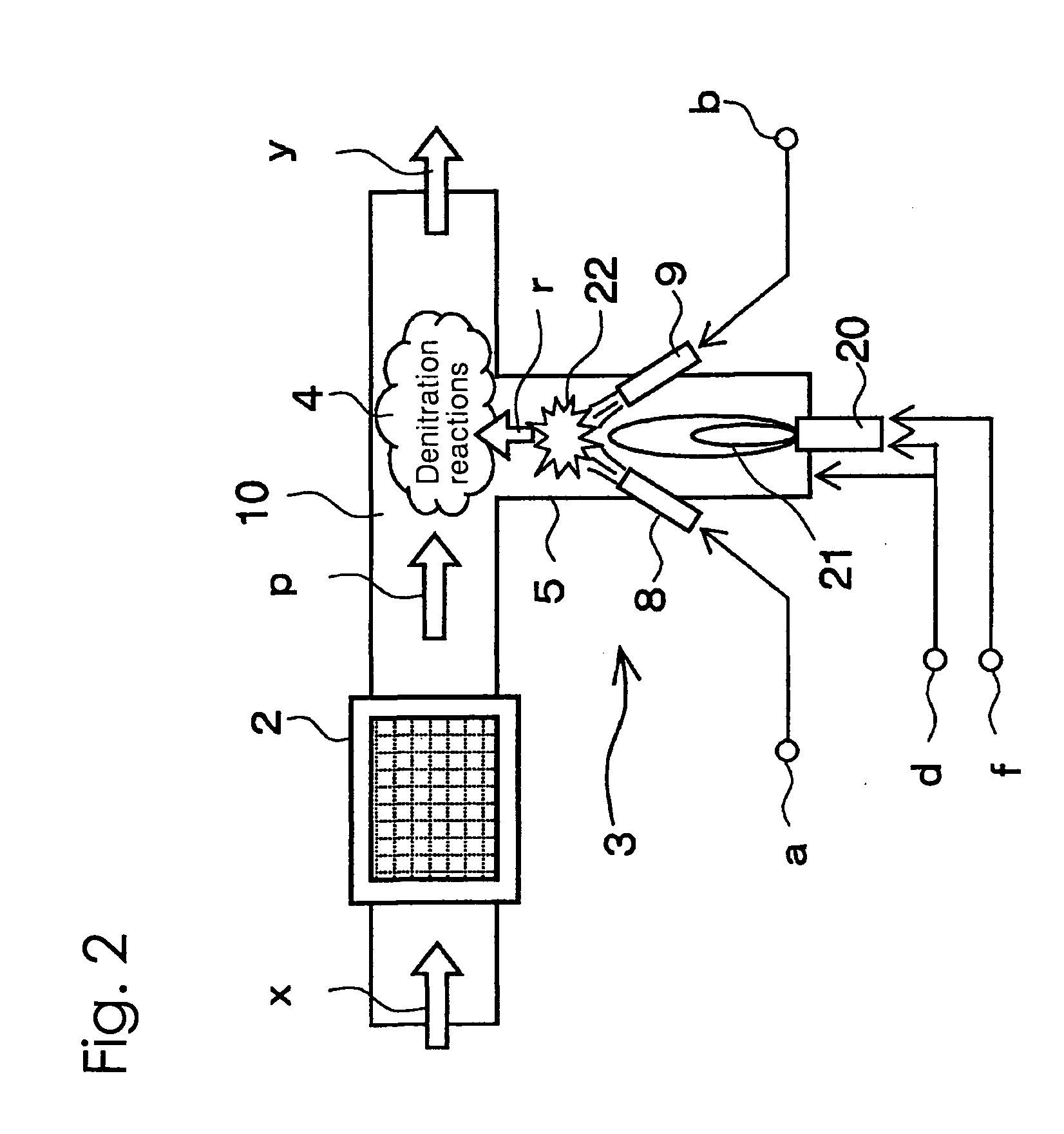
[0074]The angles formed by the center lines of the nozzle 8 and the nozzle 9 of the radical formation step 3 with the center line of the pipe portion 5 were both set to 45 degrees, and the points of intersection of the center lines of the nozzle 8 and the nozzle 9 with the center line of the pipe portion 5 were both set up so as to be positioned at a distance of 10 cm from the inner wall surface of the flue 10. In addition, the position of the burner and the strength of the flame were adjusted so as to bring the temperature of the area near the above intersection points to be 800° C. to 900° C.
[0075]A pulsed plasma generator was used as the means for the oxidation process of the preliminary step, and the denitration process was conducted so as not to activate the second denitration step. Furthermore, the exhaust gas temperature was made to be 300° C. at the measuring point 52a. Ammonia was used for the nitrogen compound blown from the nozzle 8, and propane gas was used for the hydro...
embodiment 2
[0080]The angles formed by the center lines of the nozzle 8 and the nozzle 9 of the radical formation step 3 with the center line of the pipe portion 5 were both set to 45 degrees, and the points of intersection of the center lines of the nozzle 8 and the nozzle 9 with the center line of the pipe portion 5 were both modified in the setup so as to be positioned at a distance of 5 cm from the inner wall surface of the flue 10. In addition, the position of the burner and the strenth of the flame were adjusted so as to bring the temperature of the area near the above intersection points to be 800° C. to 900° C.
[0081]The exhaust gas temperature at the measuring point 52a was made to be 250° C., NOx concentration and SO2 concentration was modified to the values shown in Table 3, and denitration treatment was conducted using a pulsed plasma generator in the preliminary step followed by a first and a second denitration step. Ammonia was used for the nitrogen compound blown from the nozzle 8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com