Grafted Photo-Polymerized Monolithic Column
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Preparation of Grafted, UV-Photopolymerized Silica-Based Monolithic Column for use in Solid Phase Extraction
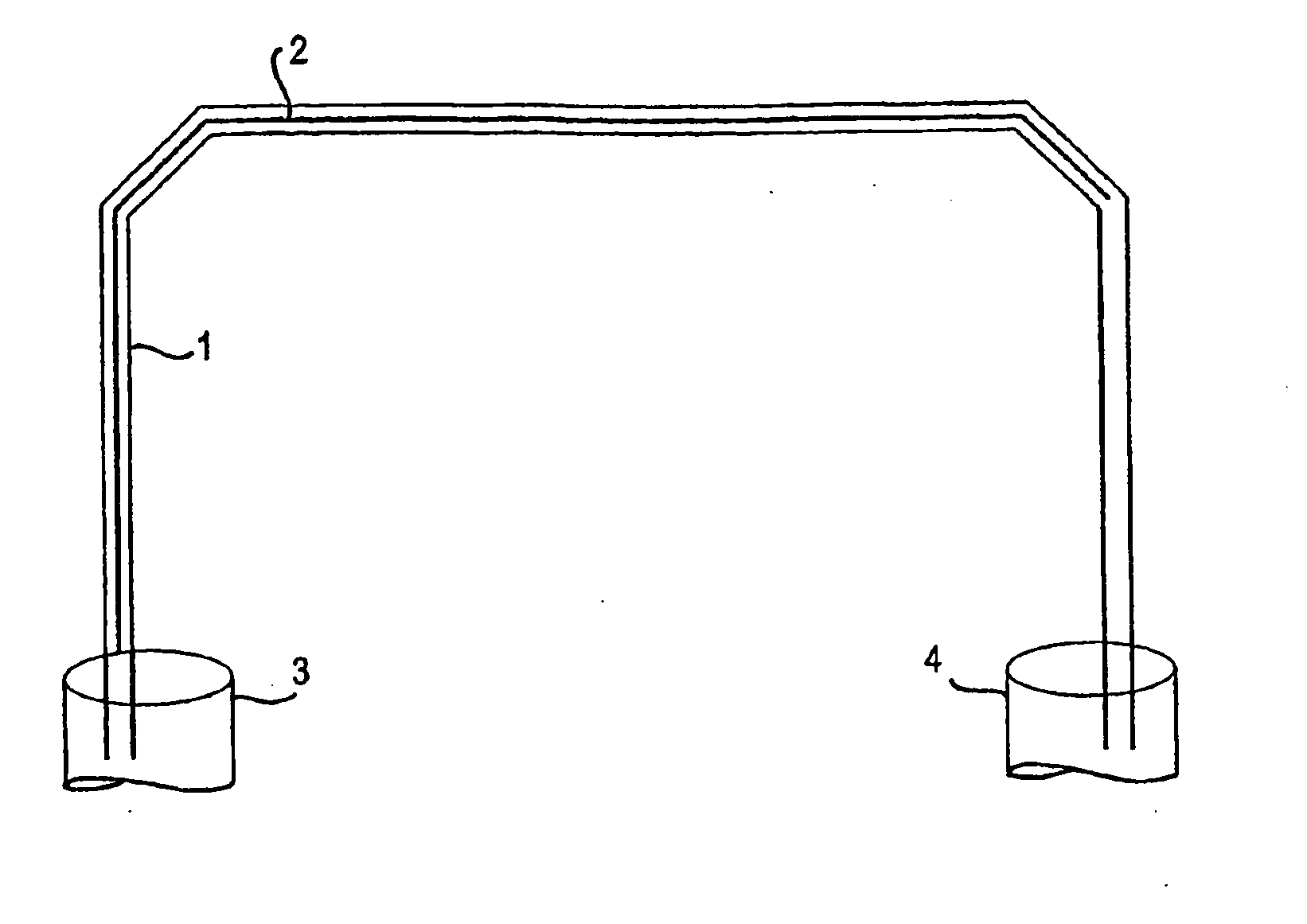
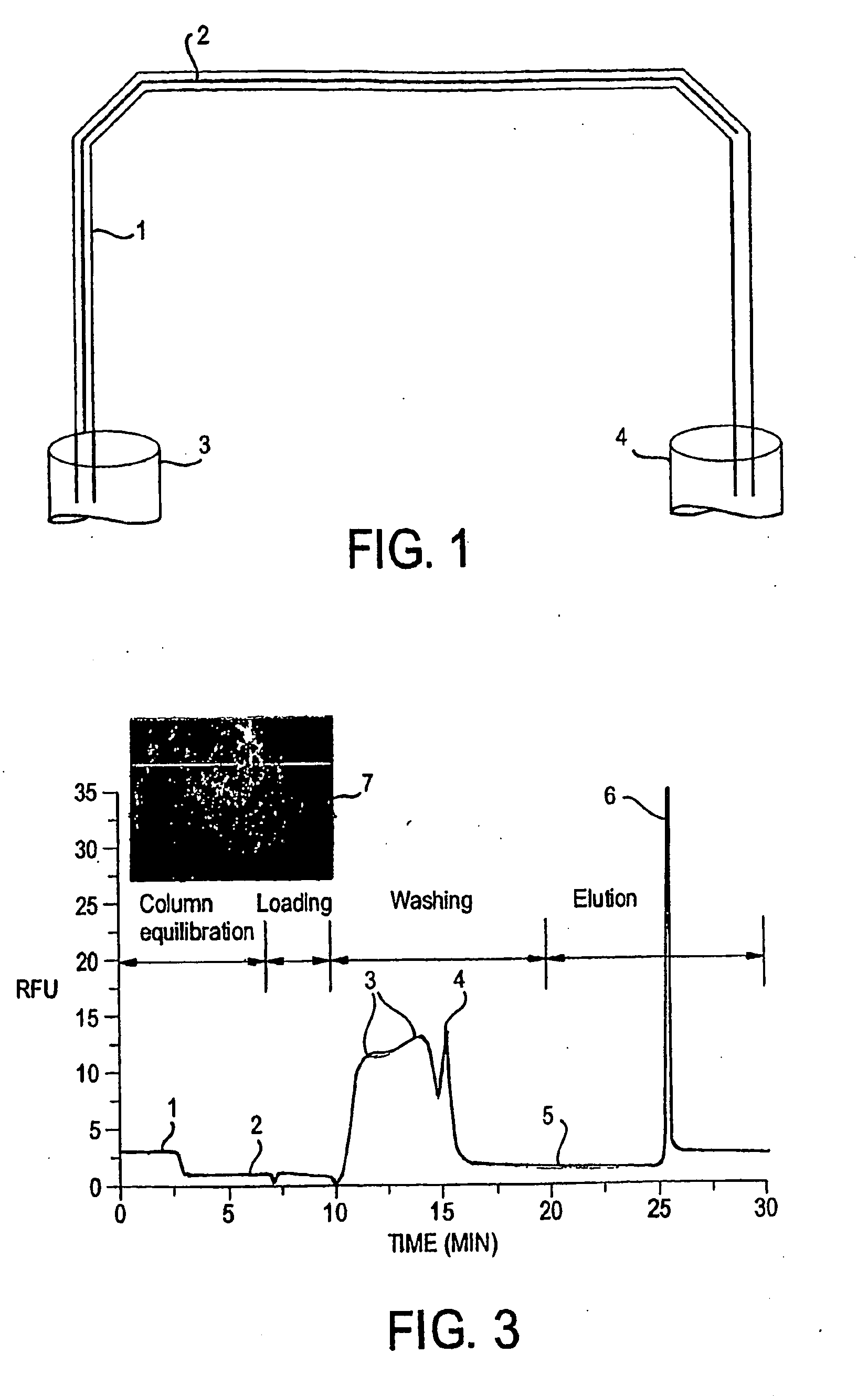
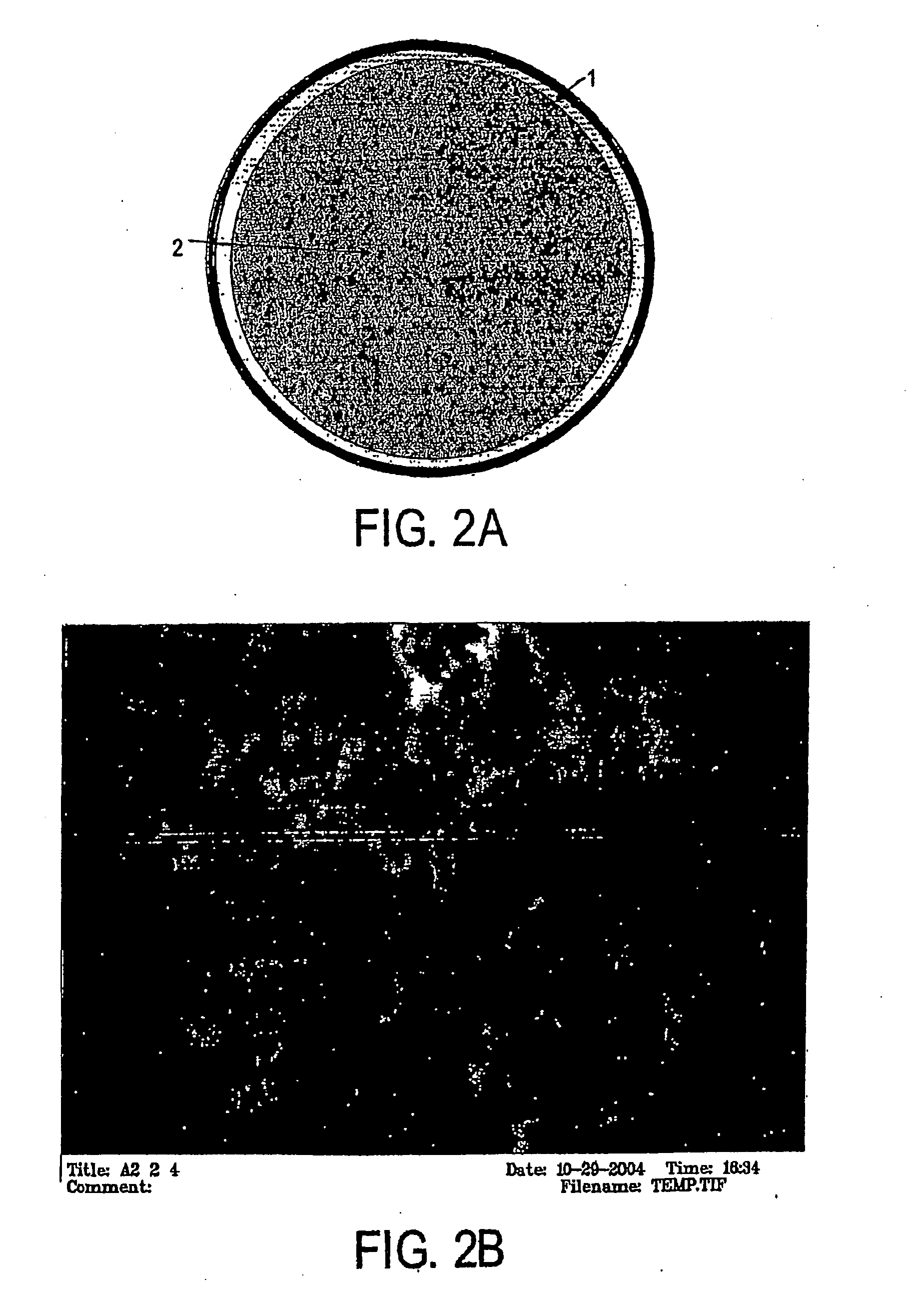
[0052]Materials and Reagents. Fused-silica capillary (250 μm i.d.×365 μm o.d.) was purchased from Supelco, Inc. (Bellefonte, Pa.). 3-(Trimethoxysilyl)propyl methacrylate (TMSPM, minimum 98%) and tetramethylorthosilicate (TMOS, 98%) were obtained from Sigma-Aldrich (Milwaukee, Wis.). Toluene (99.9%), 2-propanol (HPLC grade), guanidine hydrochloride (GuHCl, electrophoresis grade), ethanol (95%) and Tris(hydroxymethyl)aminomethane (Tris) were purchased from Fisher Scientific (Fairlawn, N.J.). EDTA was obtained from American Research Products (Solon, Ohio). Photoinitiator Irgacure 1800 was generously donated by Ciba (Tarrytown, N.Y.). All solutions were prepared with nanopure water (Barnstead / Thermolyne, Dubuque, Iowa).
[0053]Labeled DNA Fragment Generation. A 380-bp β-globin DNA fragment was amplified using the polymerase chain reaction (PCR) in a Bio-Rad Mycycle™ Thermal cycler (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com