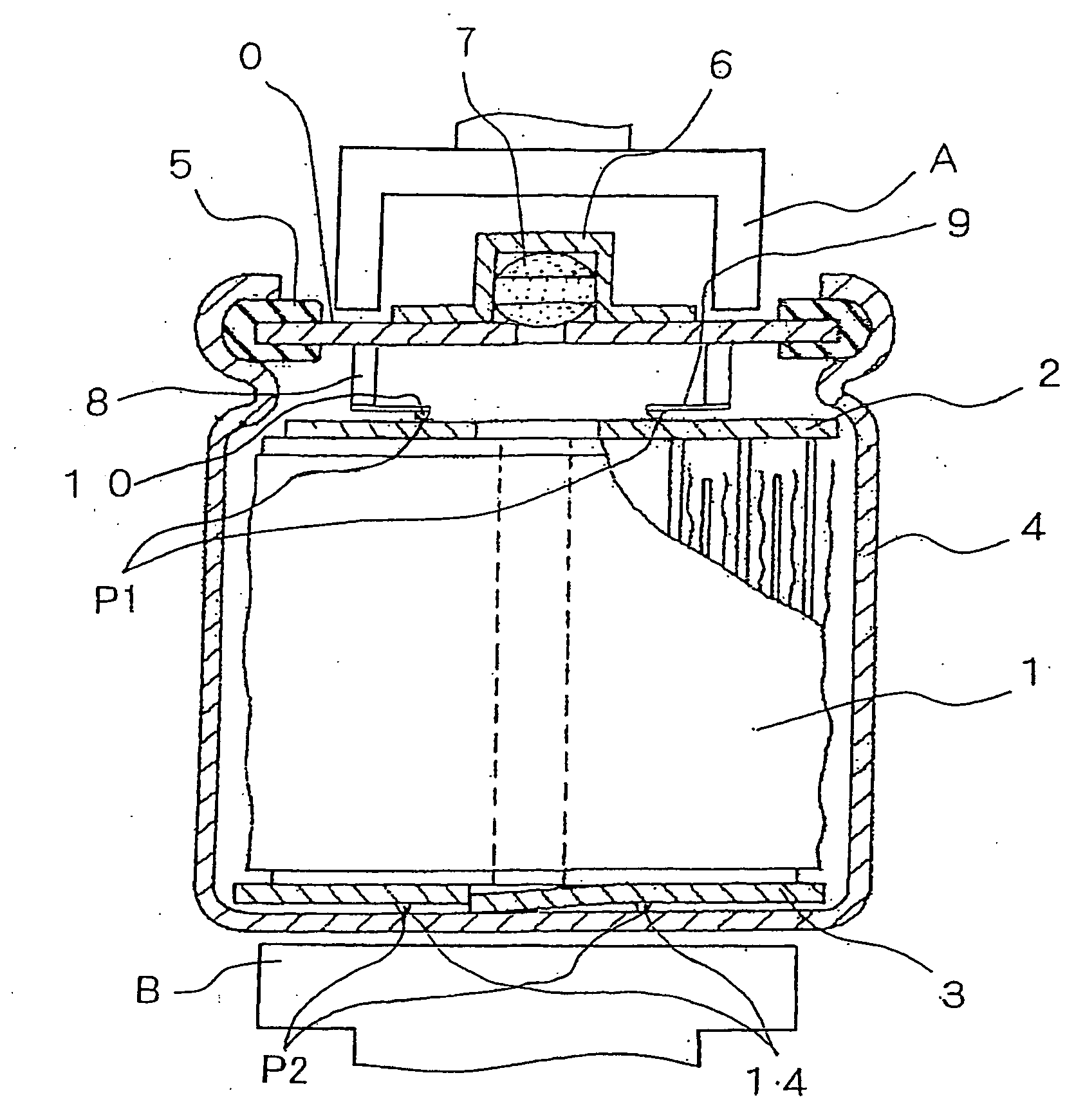
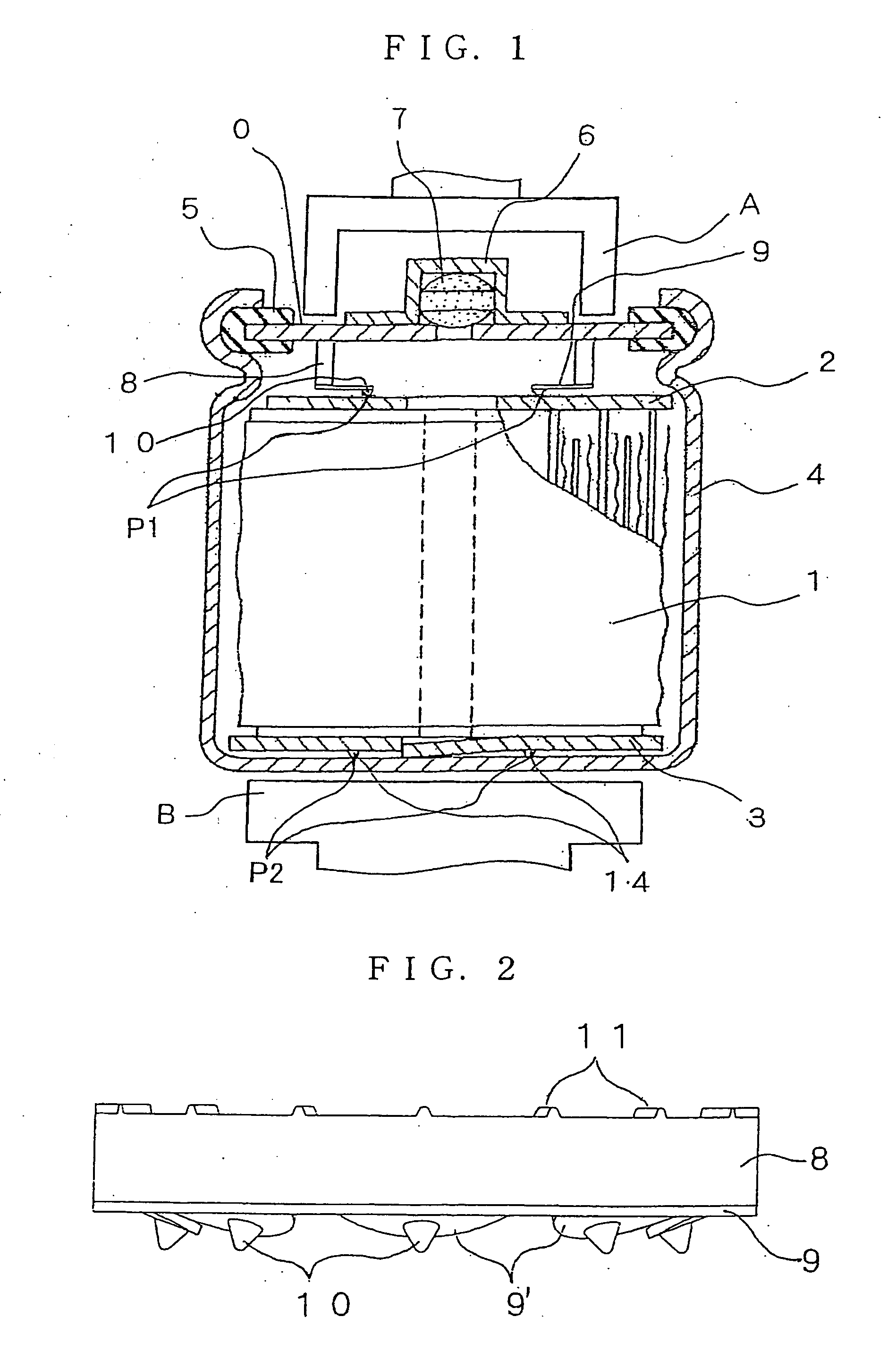
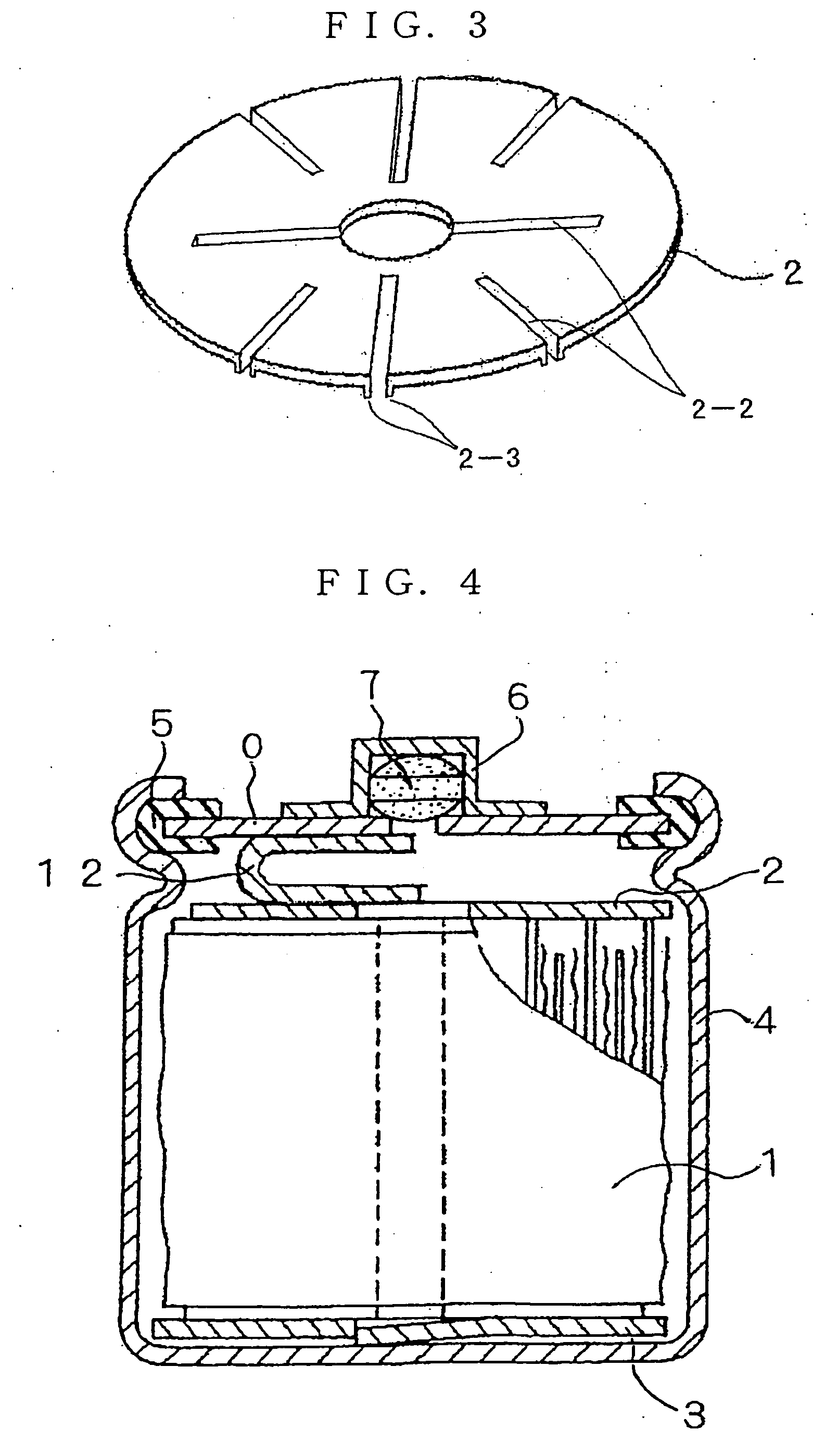
Hydrogen Absorbing Electrode and Nickel Metal-Hydridge Battery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Surface Modification Treatment of a Hydrogen Absorbing Alloy Powder)
(Pretreatment: Hydrogen Absorption)
[0130] A hydrogen absorbing alloy in the form of powder having an average diameter of 30 μm and belonging to an AB5 type rare earth element system whose composition is represented by MmNi3.55Co0.6Al0.3Mn0.35 was heated at 100° C. for 10 hours under reduced pressure. The powder was then exposed at 60° C. for 15 minutes to a hydrogen atmosphere where the partial pressure of hydrogen was kept at 0.1 Mpa, to allow the powder to absorb hydrogen.
[0131] (1st Step: Immersion Treatment)
[0132] A 1 kg of the hydrogen absorbing alloy powder treated by the hydrogen absorption treatment was immersed in 11 of an aqueous NaOH solution containing NaOH at 45 wt % and kept at 100° C. The immersion treatment was allowed to take place for 52 minutes. During the immersion treatment, the treatment solution was stirred to prevent the hydrogen absorbing powder from sinking to the bottom. During the ...
example 2
[0149] A test cell was prepared in the same manner as in Example 1, except that the immersion treatment performed in the surface modification step (1st Step: immersion treatment) for modifying the surface of a hydrogen absorbing alloy powder took 1.3 hour, and the discharge capacity of the cell was determined. The cell was made Example 2.
example 3
[0150] A test cell was prepared in the same manner as in Example 1, except that the immersion treatment performed in the surface modification step (1st Step: immersion treatment) for modifying the surface of a hydrogen absorbing alloy powder took 1.8 hour, and the discharge capacity of the cell was determined. The cell was made Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com