In vitro differentiation of vascular smooth muscle cells, methods and reagents related thereto
a vascular smooth muscle cell and in vitro technology, applied in the field of cardiovascular, can solve the problems of little knowledge about the molecular mechanisms regulating the differentiation of this cell type, and the lack of an in vitro cell differentiation system, and achieve the effect of facilitating the identification of nodal regulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro System for Differentiating Pluripotent Neural Crest Cells into Smooth Muscle Cells*
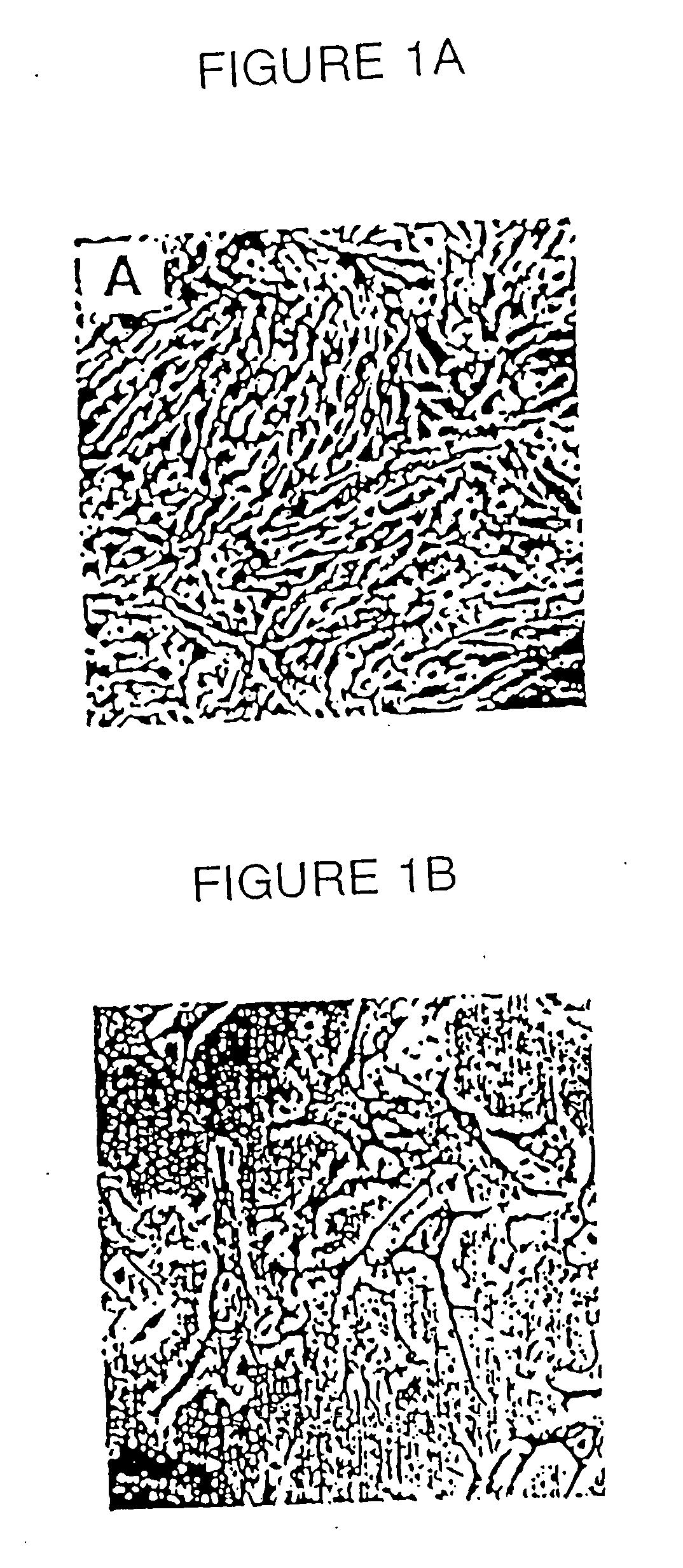
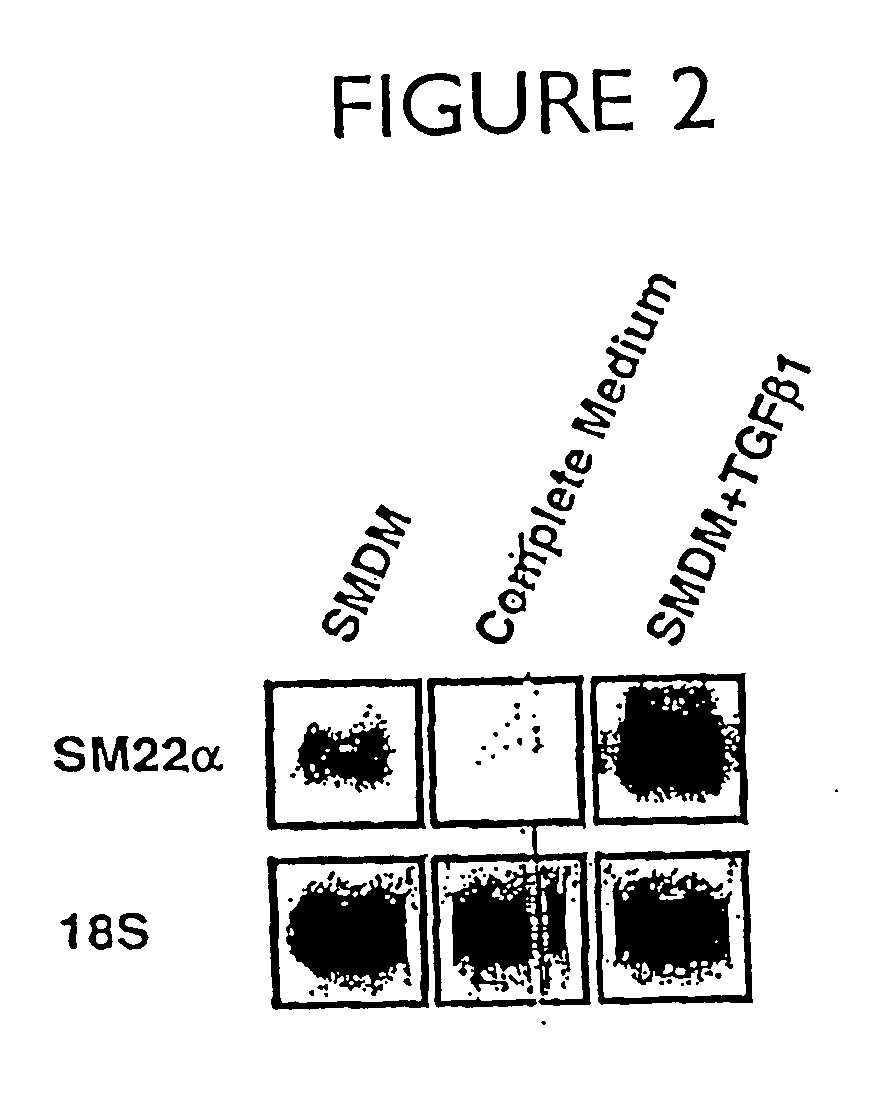
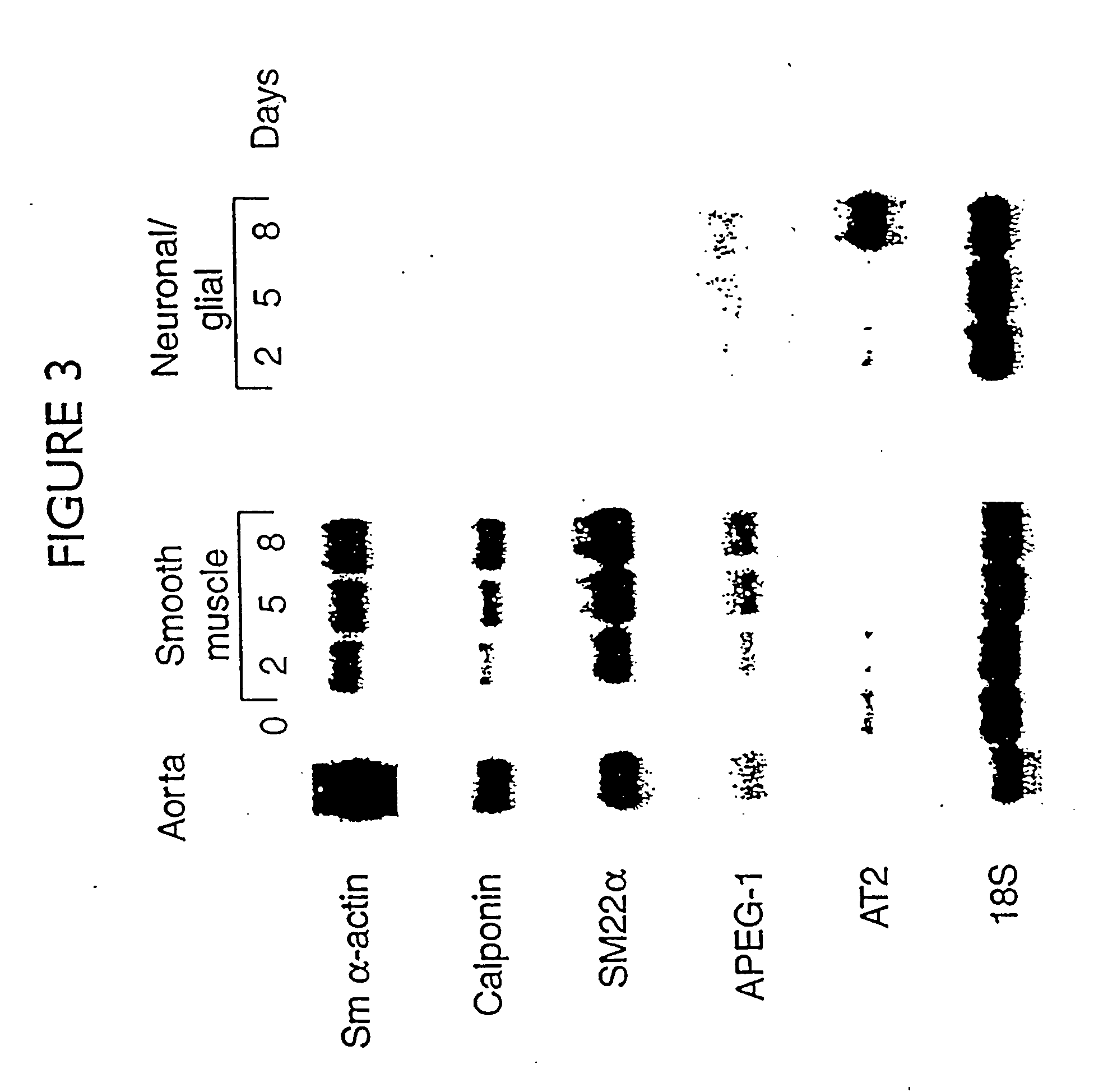
[0215] The change in vascular smooth muscle cells (SMC) from a differentiated to a dedifferentiated state is the critical phenotypic response that promotes occlusive arteriosclerotic disease. Despite its importance, research into molecular mechanisms regulating smooth muscle differentiation has been hindered by the lack of an in vitro cell differentiation system. This example identifies culture conditions that promote efficient differentiation of Monc-1 pluripotent neural crest cells into SMC. Exclusive Monc-1 to SMC differentiation was indicated by cellular morphology and time-dependent induction of the SMC markers smooth muscle -actin, smooth muscle myosin heavy chain, calponin, SM22, and APEG-1. The activity of the SM22 promoter was low in Monc-1 cells. Differentiation of these cells into SMC caused a 20-30-fold increase in the activity of the wild-type SM22 promoter and that of a hybrid...
example 2
REFERENCES FOR EXAMPLE 2
[0251] 1. Owens, G. K. (1995) Physiol. Rev. 75, 487-517 2. Ross, R. (1993) Nature 362, 801-809 3. Kirby, M. L., and Waldo, K. L. (1995) Circ. Res. 77,211-215 4. Kirby, M. L., and Waldo, K. L. (1990) Circulation 82, 332-340 5. Noden, D. M. (1989) Am. Rev. Respir. Dis. 140, 1097-1103 6. Topouzis, S., and Majesky, M. W. (1996) Dev. Biol. 178, 430-445 7. Gunther, S., Alexander, R. W., Atkinson, W. J., and Gimbrone, M. A., Jr. (1982) J. Cell. Biol. 92, 289-298 8. Stemple, D. L., and Anderson, D. J. (1992) Cell 71, 973-985 9. Jain, M. K., Layne, M. D., Watanabe, M., Chin, M. T., Feinberg, M. W., Sibinga, N. E. S., Hsieh, C.-M., Yet, S.-F., Stemple, D. L., and Lee, M.-E. (1998) J. Biol. Chem. 273, 5993-5996 10. Blanar, M. A., and Rutter, W. J. (1992) Science 256, 1014-1018 11. Blanar, M. A., Crossley, P. H., Peters, K. G., Steingrimsson, E., Copeland, N. G., Jenkins, N. A., Martin, G. R., and Rutter, W. J. (1995) Proc. Natl. Acad. Sci. U. S. A. 92, 5870-5874 12. He,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com