Mutants of green fluorescent protein
a technology of green fluorescent protein and mutants, which is applied in the field of molecular and cellular biology, can solve the problems of only useful type of technique, inability to guarantee that a majority (or even any) of the target cells will take up and/or express exogenous dna, and reporter genes typically do not confer any particular advantage to the recipient, etc., and achieves the effect of being convenient to us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Mutant GFP cDNAs
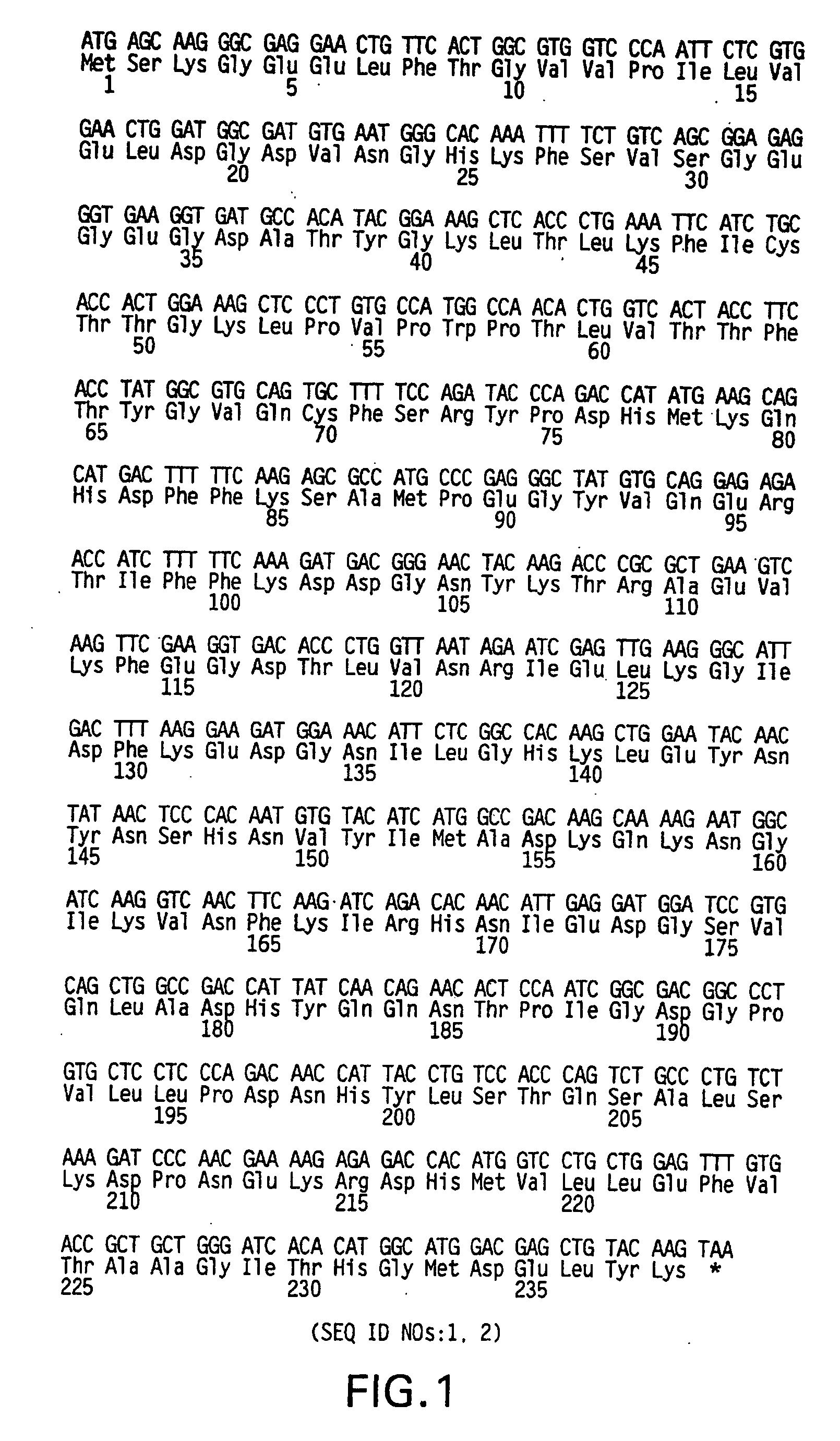
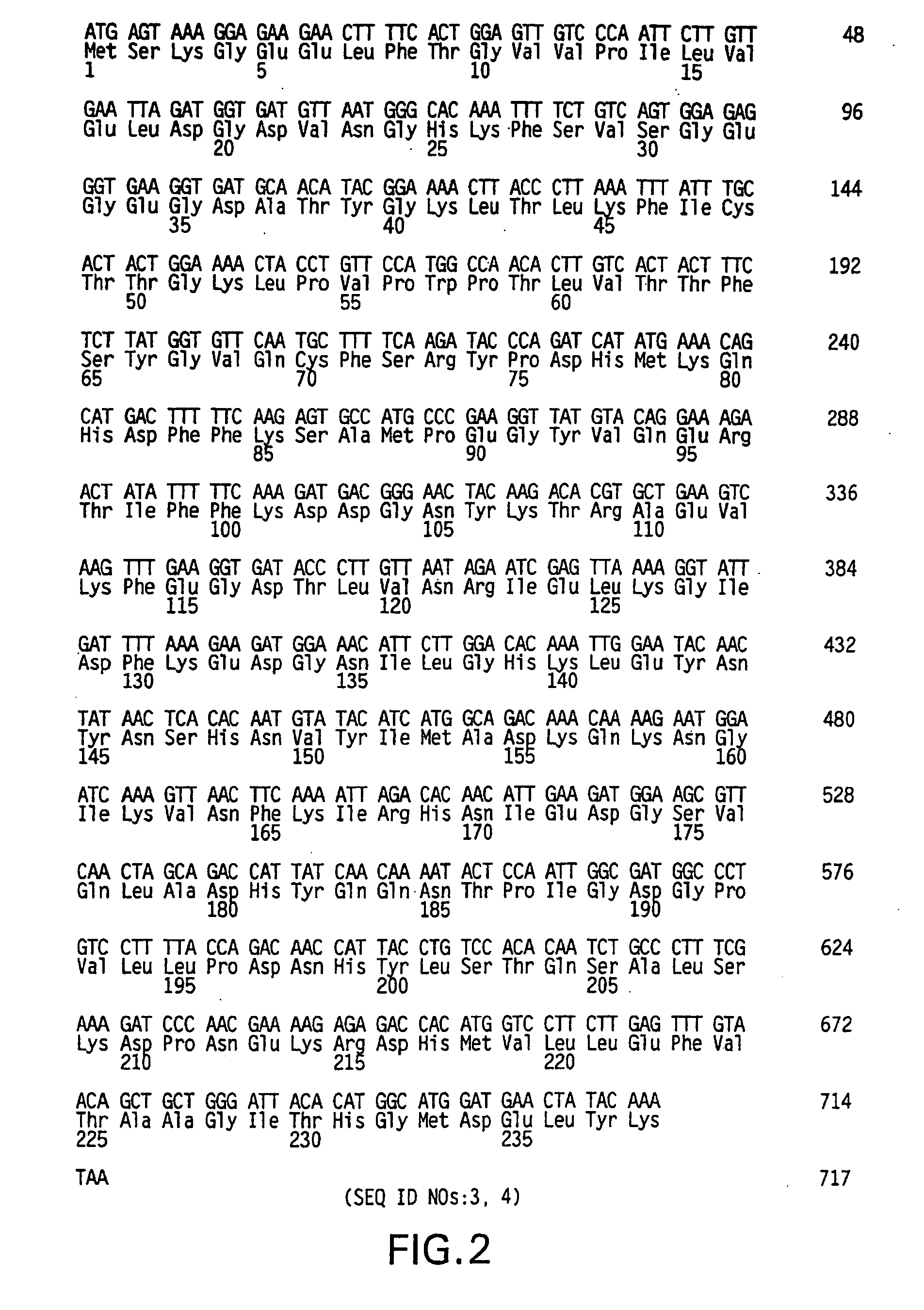
[0080] Plasmids. As depicted in FIG. 5, pGreenLantern-1 (Invitrogen Corporation, Carlsbad, Calif.; catalogue no. 10642) contains the humanized S65T mutant GFP cDNA (FIG. 1; SEQ ID NOs:1, 2) (Evans, K., et al., FOCUS 18(2):40-43 (1996); Zolotukhin, S., et al., J. Virol. 70:4646-4654 (1996)). This plasmid serves as the source of the GFP DNA sequence used for mutagenesis. As depicted in FIG. 6, pGreenLantern-2 contains a universal sequencing primer downstream of the CMV promoter along with an NsiI site immediately upstream of the CMV promoter allowing excision of the promoter region. It also contains XbaI, XhoI and HindIII sites in place of the 3′ NotI site in pGreenLantern-1. A stop codon in the 5′ multiple cloning site of pGreenLantern-1 was shifted out of frame to allow possible fusions to GFP in pGreenLantern-2.
[0081] Mutations to GFP cDNA by UDG cloning. PCR was performed in an MJ Research DNA Engine™ thermal cycler using the following conditions:...
example 2
Growth and Transfection of Host Cells With Mutant GFPs
[0083] Cell Culture. Chinese hamster ovary cells (CHO-K1, obtained from American Type Culture Collection (ATCC), Rockville, Md.) were cultured in D-MEM (4,500 milligrams / liter D-glucose with L-glutamine and phenol red) plus 10% fetal bovine serum (FBS), 0.1 millimolar nonessential amino acids, 2.5 units per milliliter penicillin and 2.5 micrograms per milliliter streptomycin (Freshney, R. I., Culture of Animal Cells: A Manual of Basic Techniques, 3rd Ed., New York: Wiley-Liss (1994)). Cells were grown at 37° C. in a 5% CO2 / air incubator. All media and reagents were from Invitrogen Corporation, Carlsbad, Calif.
[0084] Transfection. CHO-K1 cells were plated at 2×105 cells per well into six-well (35 millimeter diameter) plates one day prior to transfection. Immediately before transfection, cells were rinsed with medium containing no serum or antibiotics. LIPOFECTAMINE™ reagent was diluted into 100 microliters of OPTI-MEM-I Reduced ...
example 3
Characterization of GFP Mutants Expressed in Eukaryotic Cells
[0086] Formalin Fixation. Transfected host cells were rinsed in Dulbecco's Phosphate Buffered Saline (PBS), then fixed in a solution of 10% formalin in PBS for one hour. Formalin was then removed, and cells were rinsed and stored in PBS at 4° C. until being analyzed.
[0087] Fluorescence Microscopy. Formalin-fixed cells were examined and photographed using an inverted phase contrast fluorescence microscope equipped with FITC filters (excitation 475 nm / dichroic 485 nm / barrier 490 nm) and a 50 watt mercury arc bulb at 1.25 volts. A 40×-power adjustable non-phase objective was used for all micrographs, which were taken through blue, neutral and FITC filters using Kodak Ektachrome ASA 400 Daylight (for slides) or Kodak Gold ASA 400 Daylight (for prints). All exposures were for 12 seconds to allow unbiased comparison of fluorescence intensity.
[0088] Flow Cytofluorimetry. Flow cytofluorimetry was performed on transfected CHO-K1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com