Cell patterning technique
a cell patterning and cell technology, applied in the field of cell patterning technique, can solve the problems of destroying the cells themselves, requiring clean-room facilities and other complex equipment, and not being readily accessible to most biologists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fabrication of Masking System
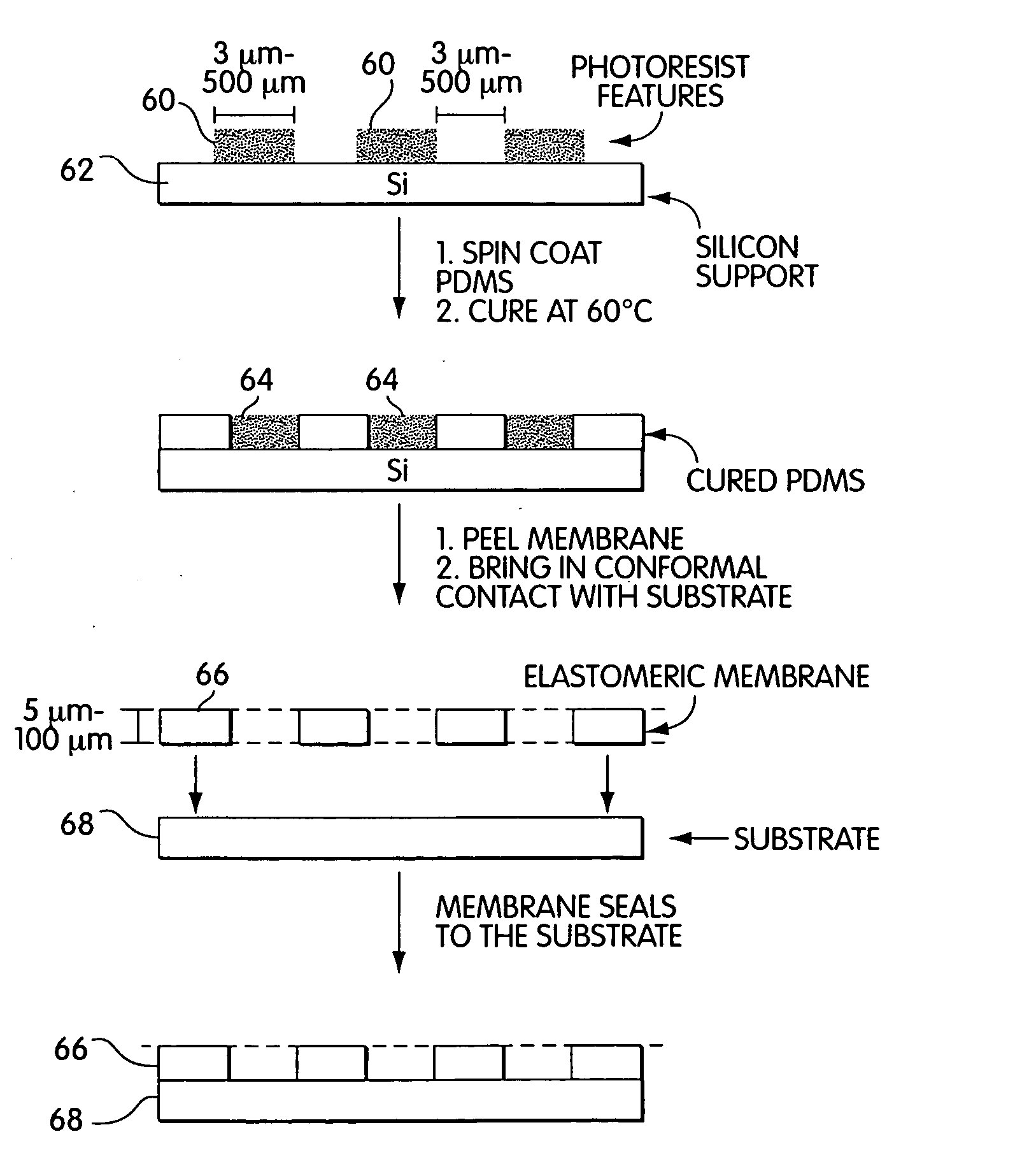
[0067] Fabrication of Patterned Photoresist Structures and Membranes. With reference to FIG. 3, arrays of cylindrical posts of photoresist 60 were fabricated on silicon wafers 62 using standard photolithographic techniques and rigid chrome masks. Arrays of square features were fabricated using transparencies as photomasks. We used procedures well-known in the art to fabricate features there were 50 μm high.
[0068] Fabrication of Masking Systems. Elastomeric polymer membranes were fabricated using the procedure described by Jackman et al. (Jackman et al., Langmuir, vol. 15, pp. 2973-2984, (1999)). The PDMS prepolymer 64 (mixed in a 10:1 ratio with a crosslinking catalyst) was spin-coated on the bas-relief of patterned photoresist using parameters known to produce a film that was thinner than the height of the features of photoresist. For features that were 50 μm tall, we spin-coated PDMS prepolymer at 3000 rpm for 60 sec to generate a film that was appro...
example 2
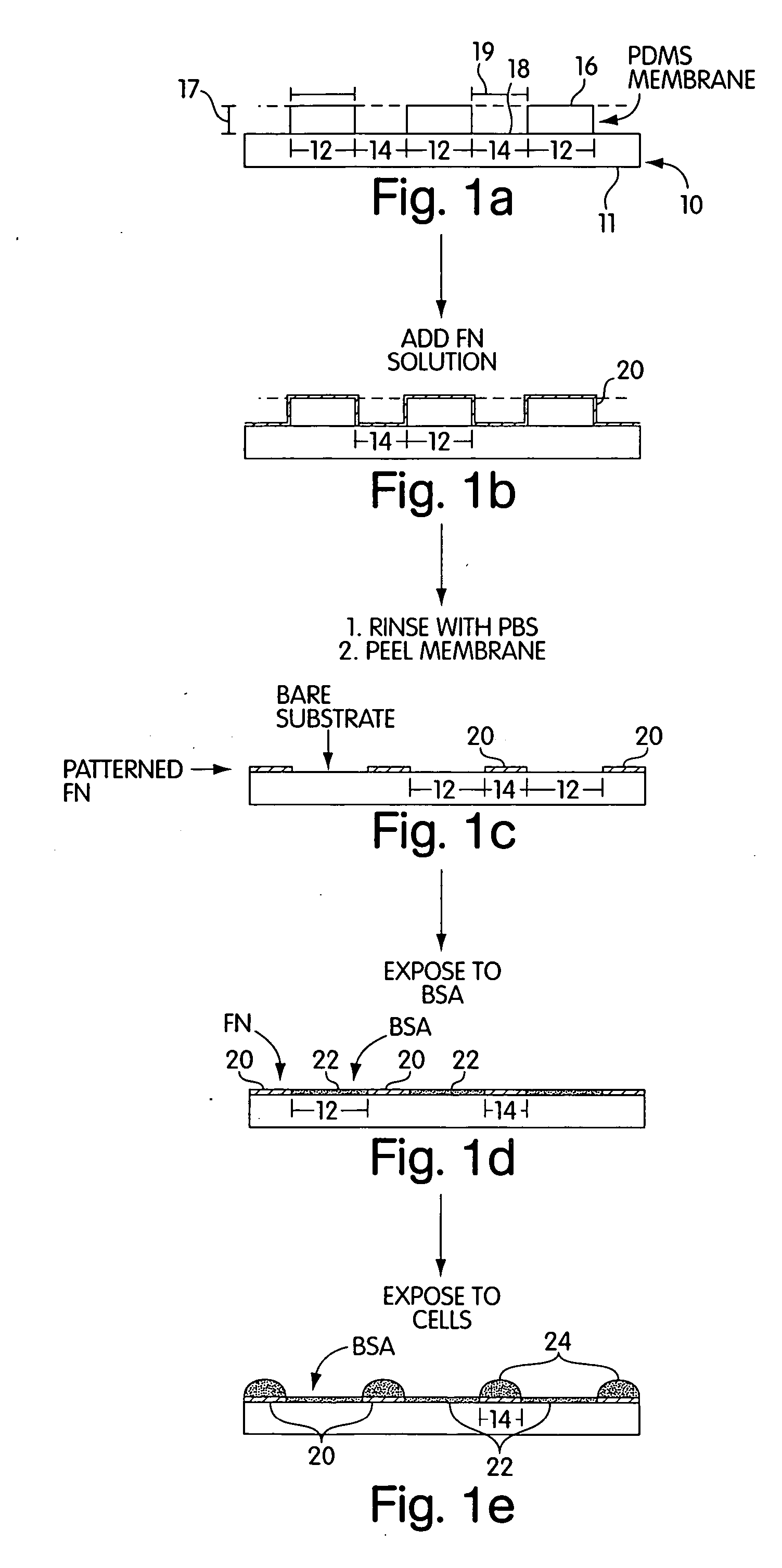
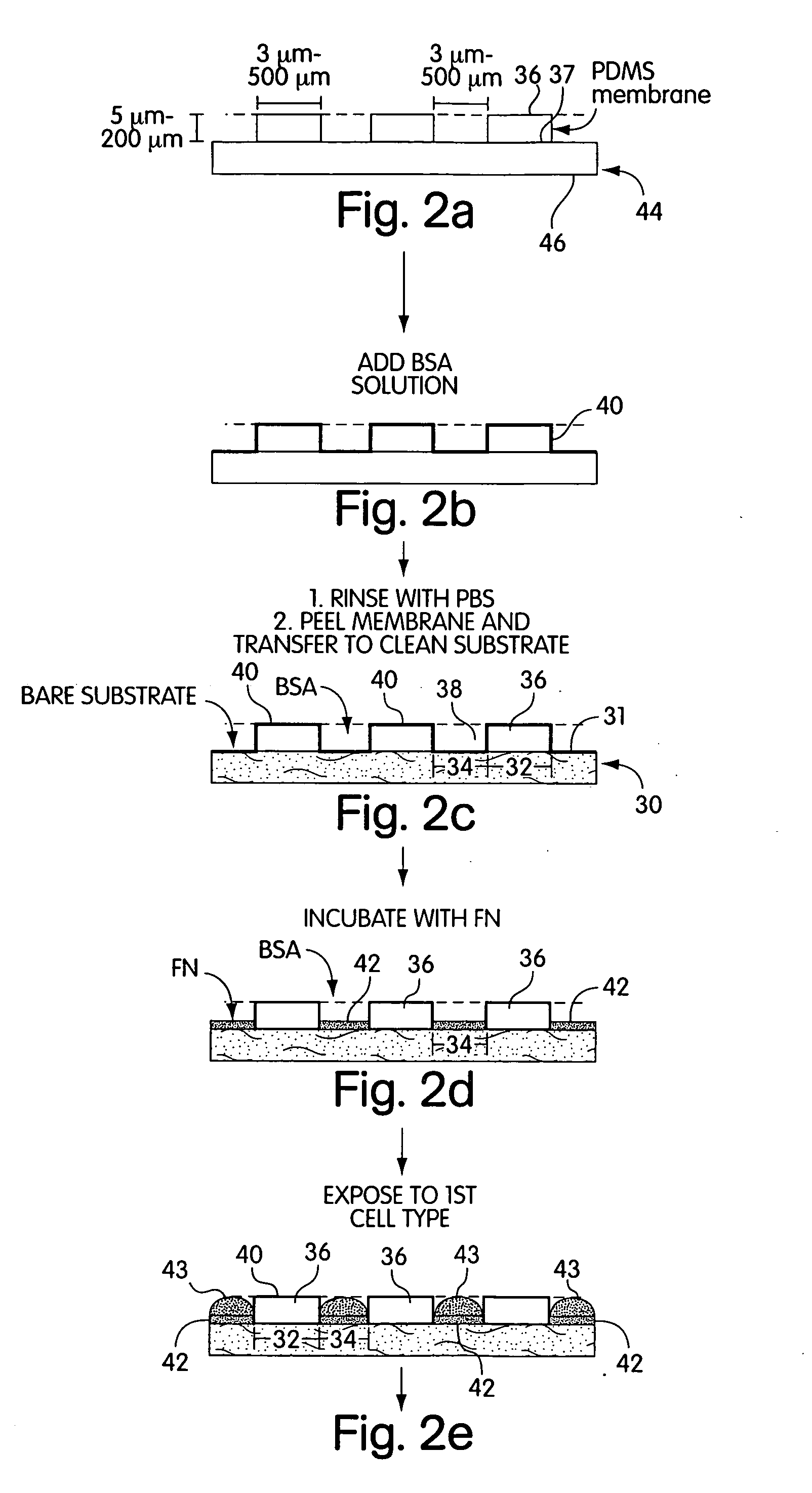
[0071] a) Pre-coating the membrane with a cell adhesion inhibitor. In a laminar flow hood, the membranes were placed on the surface of a sterile Petri dish with a few drops of ethanol. The liquid sterilized the membranes by killing bacteria. Drops of a buffered solution of BSA (1% w / v, in PBS or DMEM at pH=7.4) were placed on the membrane to cover the holes, in a manner schematically described in FIG. 2. Since the liquid did not fill the hydrophobic pores, vacuum was applied (˜30 sec) and released (˜500 mTorr) twice to extract the air trapped in the pores. BSA was allowed to adsorb to the surfaces for 15 min. The substrates were then rinsed three times with PBS; the membranes were peeled from the support in the presence of PBS, and transferred to a clean Petri dish covered with PBS to help seal the membrane onto the dish.
[0072] b) Patterning Proteins on Substrates. Drops of a cell-adhesion promoter, buffered fibronectin (50 μg / mL, PBS with pH=7.4) or gelatin (1...
example 3
Cell culture
[0074] a) Growth and attachment. Bovine adrenal capillary endothelial (BCE) cells were cultured under 10% CO2 on cell culture Petri dishes (Falcon) coated with gelatin in DMEM containing 10% calf serum, 2 mM glutamine, 100 μg / mL streptomycin, 100 μg / mL penicillin, and 1 ng / mL basic fibroblast growth factor (bFGF).2 Prior to incubation with the patterned substrates prepared using MEMPAT, cells were dissociated from culture plates with trypsin-EDTA and washed in DMEM containing 1% BSA (BSA / DMEM). The suspension of cells (typically 25,000 cells / mL, 2 mL total volume) was placed on the substrates in chemically defined medium (10 μg / mL high density lipoprotein, 5 μg / mL transferrin, 5 ng / mL basic fibroblast growth factor in 1% BSA / DMEM) and incubated in 10% CO2 at 37° C.
[0075] b) Fixing and Staining Cells. Substrates that contained cells were fixed with PFA for 20 min and washed with PBS. The substrates were then washed with methanol for 1 min, and stained with Coomassie Blu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com