Implantable devices and methods for treatment of pain by delivery of fentanyl and fentanyl congeners
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment Regimen for Subcutaneous Delivery of Fentanyl or Fentanyl Congener
[0138] The following is an exemplary treatment regimen. The approximate volume rate of release may be about 1.5 microliters per hour, with approximately zero order kinetics, for over 3 days.
[0139] 1. Evaluation of Patient.
[0140] The physician first examines the potential patient and evaluates the patient's history to determine if the patient has pain that amenable to treatment by fentanyl and congeners and can safely tolerate such treatment.
[0141] 2. Selection of Appropriate Dose.
[0142] If the physician decides to proceed with treatment in accordance with this invention, the physician determines the appropriate dose of drug (e.g., sufentanil or fentanyl) to be administered to the patient. This determination can be performed in a variety of ways.
[0143] If the patient is already using medication to control pain (e.g., oral morphine or the fentanyl transdermal patch), the physician can correlate the dose ...
example 2
Drug Loading for Device Comprising an Osmotic Pump
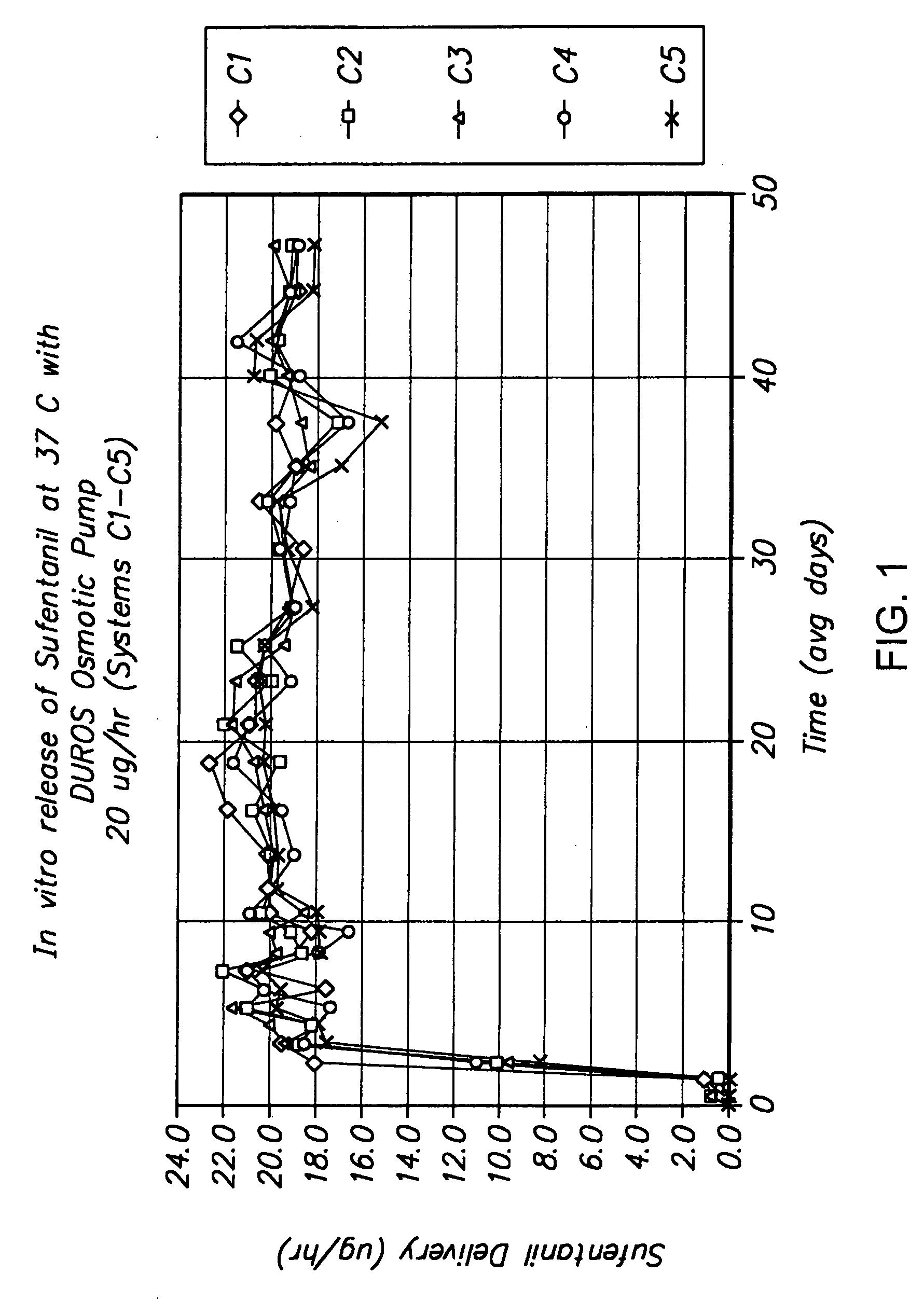
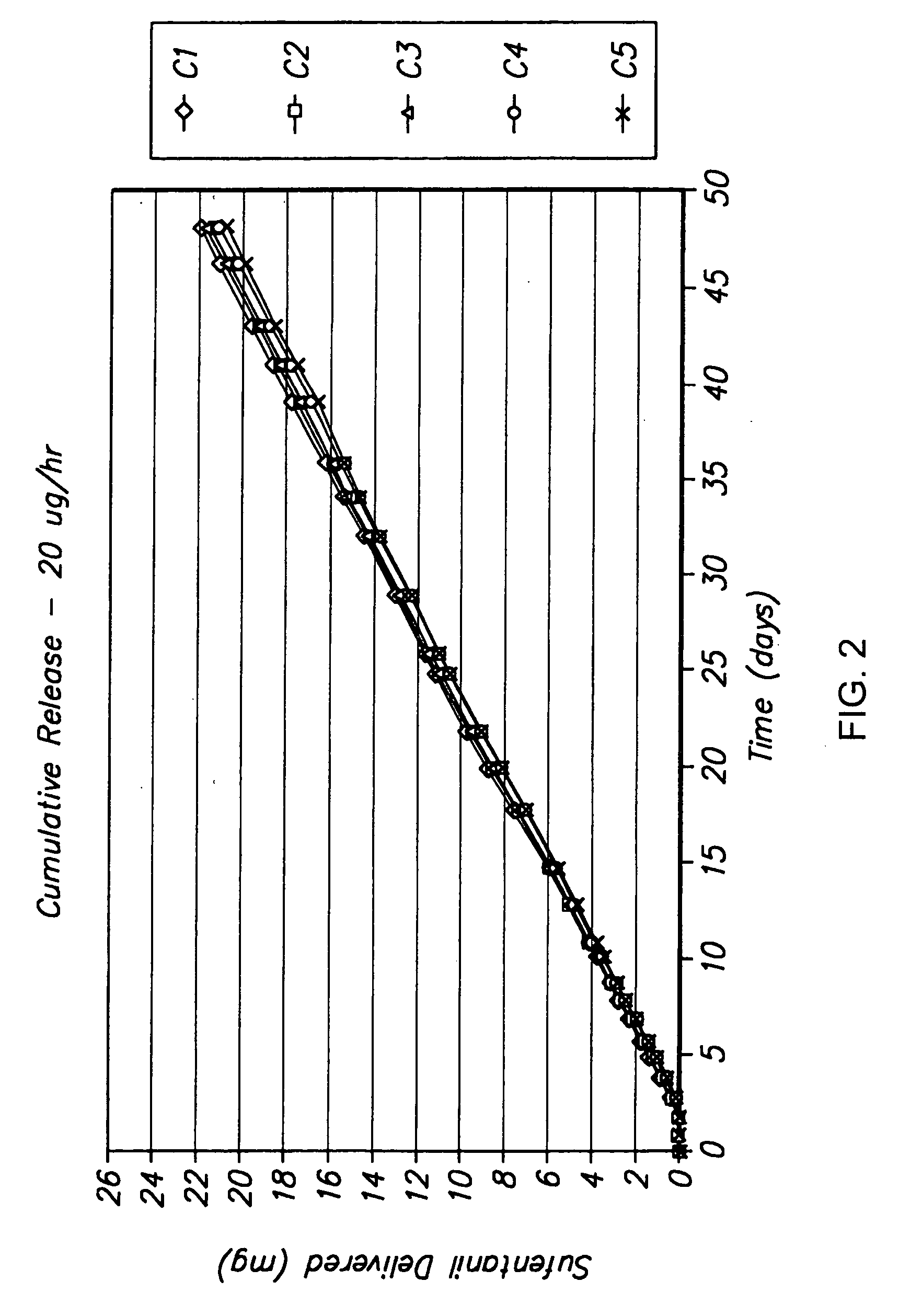
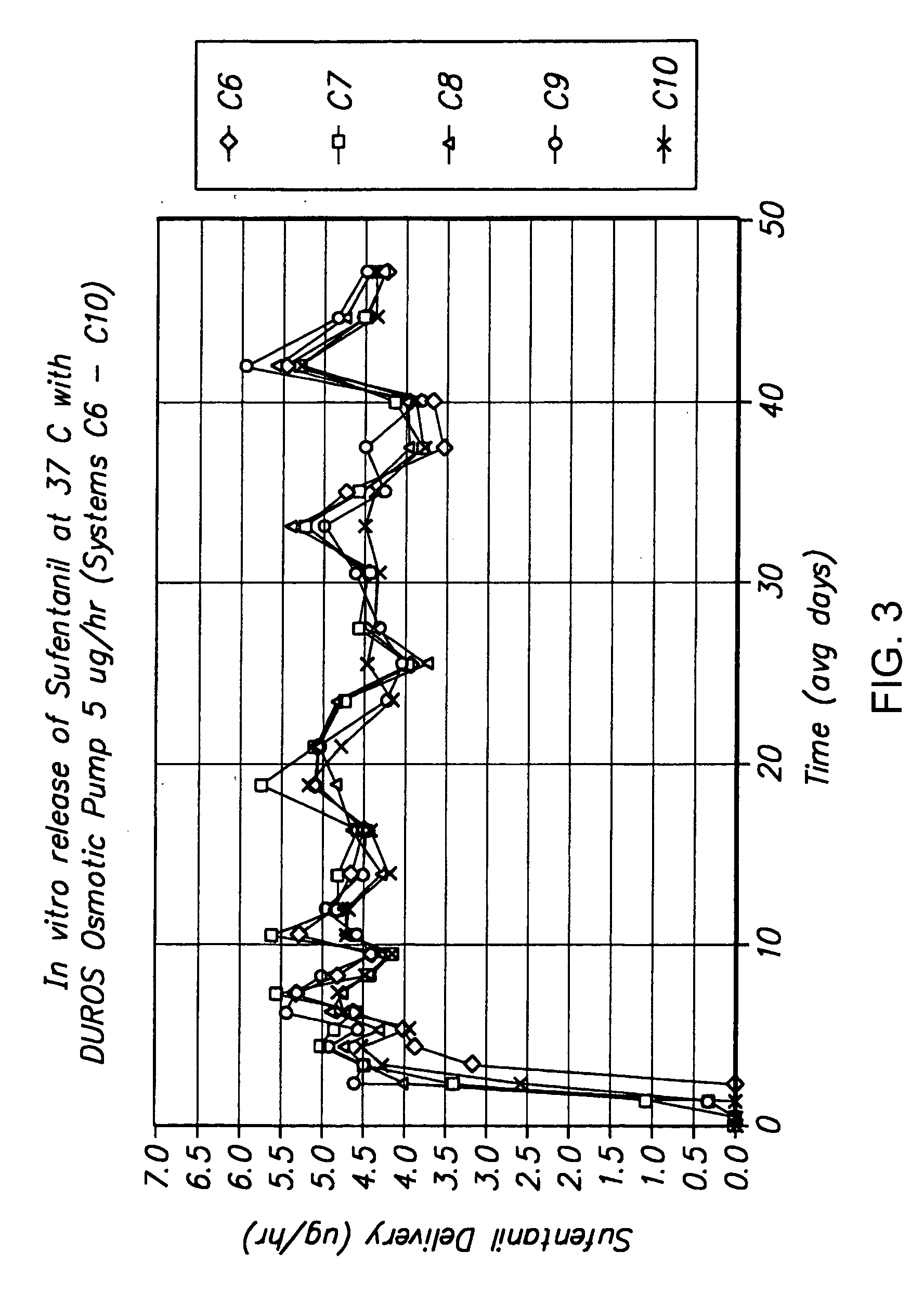
[0152] The following is a description of exemplary drug loading parameters of a device comprising an osmotic pump used for the delivery of sufentanil. In this example, the thermal expansion element of the device comprised a plug as illustrated in FIG. 7, which plug defines an inlet, an expansion control channel and an outlet. At least a portion of the delivery outlet is defined by the plug and an inner wall of housing 50. As shown in FIG. 7, plug 600 is inserted in housing 50 and is in fluid communication with formulation 740 contained in reservoir 700 of drug delivery device 680. Plug 600 comprises inner plug member 610 positioned within outer plug member 750. Expansion control channel 620 extends longitudinally from a first end of inner plug member 610 (which end defines inlet 650) and through the body of the inner plug member; a laterally extending section, which extends from the body of the inner plug member 610 and to an outer ...
example 3
Formulations Comprising Sufentanil in Benzyl Alcohol
[0155] 397 mg / mL formulation: 3.97 g of sufentanil base were weighed out and added to a portion of benzyl alcohol. The drug was dissolved in the benzyl alcohol by stirring with a magnetic stirrer. When the resultant preparation was clear, additional benzyl alcohol was added to obtain 10 mL of formulation. The resultant formulation concentration was 397 mg / mL.
[0156] 310 mg / mL formulation: 3.10 g of sufentanil base were weighed out and added to a portion of benzyl alcohol. The drug was dissolved in the benzyl alcohol by stirring with a magnetic stirrer. When the resultant preparation was clear, additional benzyl alcohol was added to obtain 10 mL of formulation. The resultant formulation concentration was 310 mg / mL.
[0157] 208 mg / mL formulation: 2.08 g of sufentanil base were weighed out and added to a portion of benzyl alcohol. The drug was dissolved in the benzyl alcohol by stirring with a magnetic stirrer. When the resultant prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com