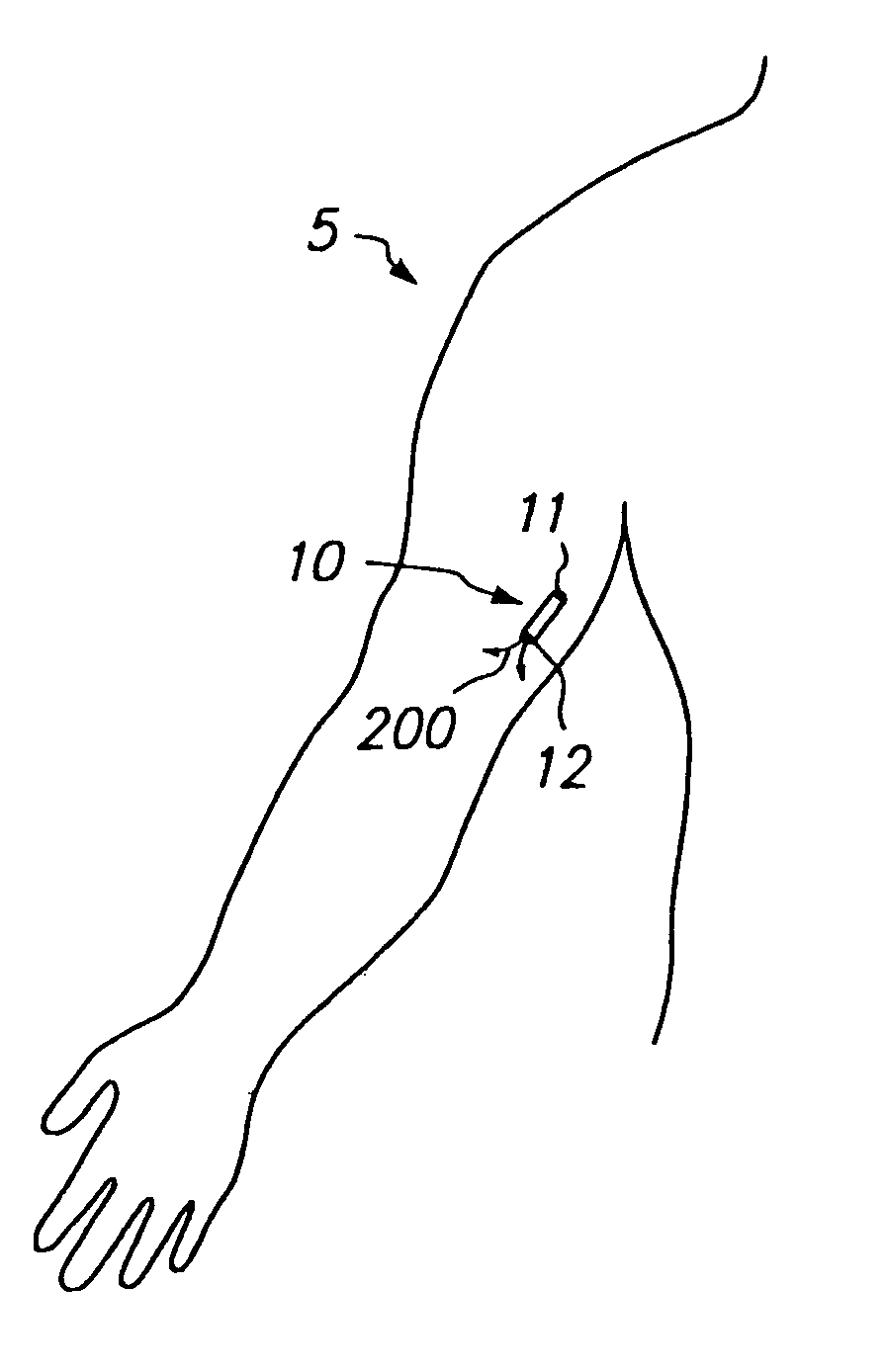
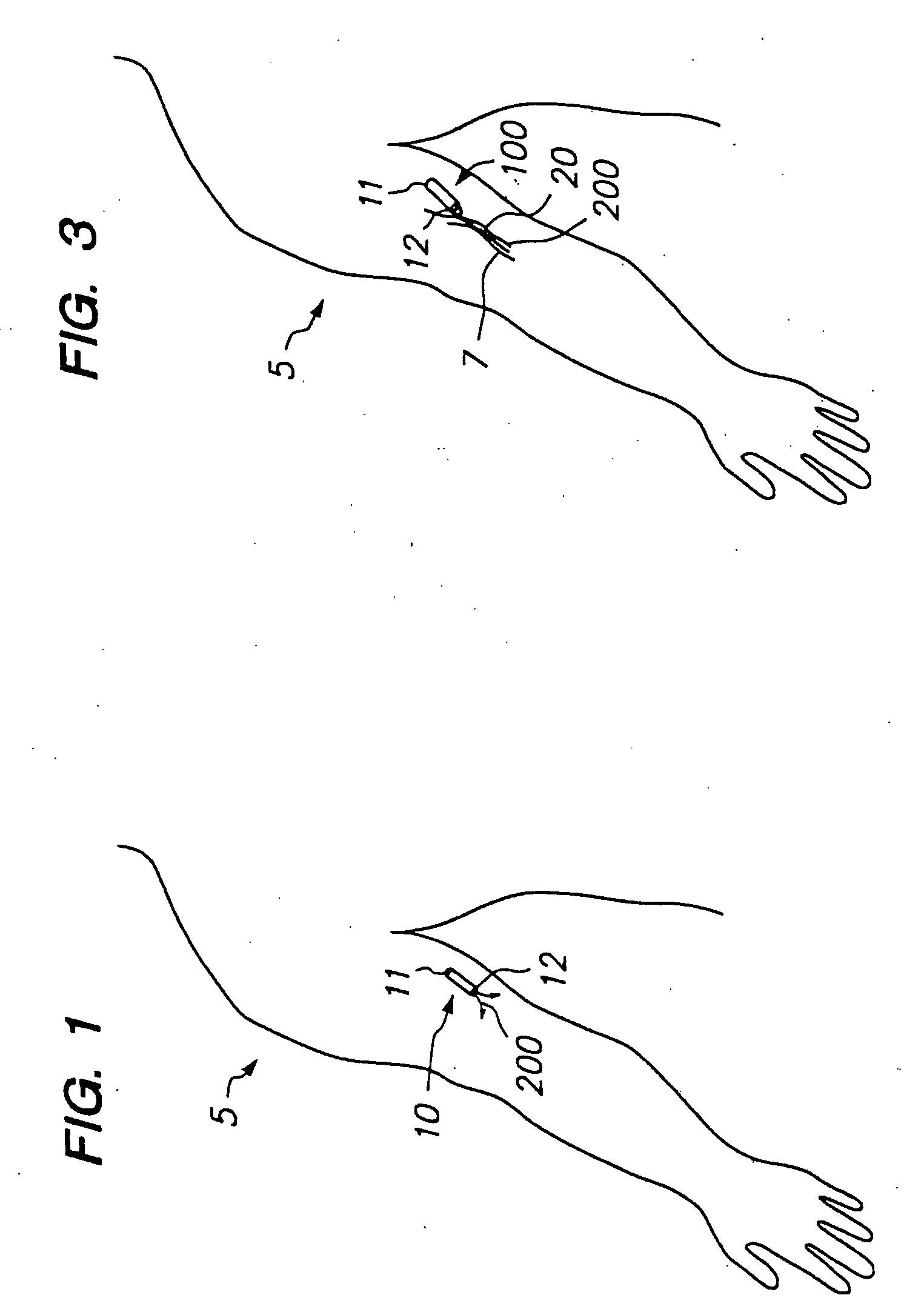
Devices and methods for pain management
a technology for pain management and devices, applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of opiate tolerance, dependence, and dependence, and achieve the effects of adequate pain relief, effective pain management, and improved adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment Regimen for Subcutaneous Delivery of Fentanyl or Fentanyl Congener Via DUROS™ Pump:
[0110]1. Evaluation of Patient.
[0111]The physician first examines the potential patient and evaluates the patient's history to determine if the patient has pain that amenable to treatment by opioids and can safely tolerate such treatment.
[0112]2. Selection of Appropriate Dose.
[0113]If the physician decides to proceed with treatment in accordance with this invention, the physician determines the appropriate dose of drug (e.g., sufentanil) to be administered to the patient. This determination can be performed in a variety of ways. If the patient is already using certain medication to control pain (e.g., oral morphine or the fentanyl transdermal patch), the physician can attempt to correlate the dose of medication previously used by the patient to an appropriate dose of the selected drug (e.g., fentanyl or fentanyl congener such as sufentanil) when infused subcutaneously. This correlation can b...
example 2
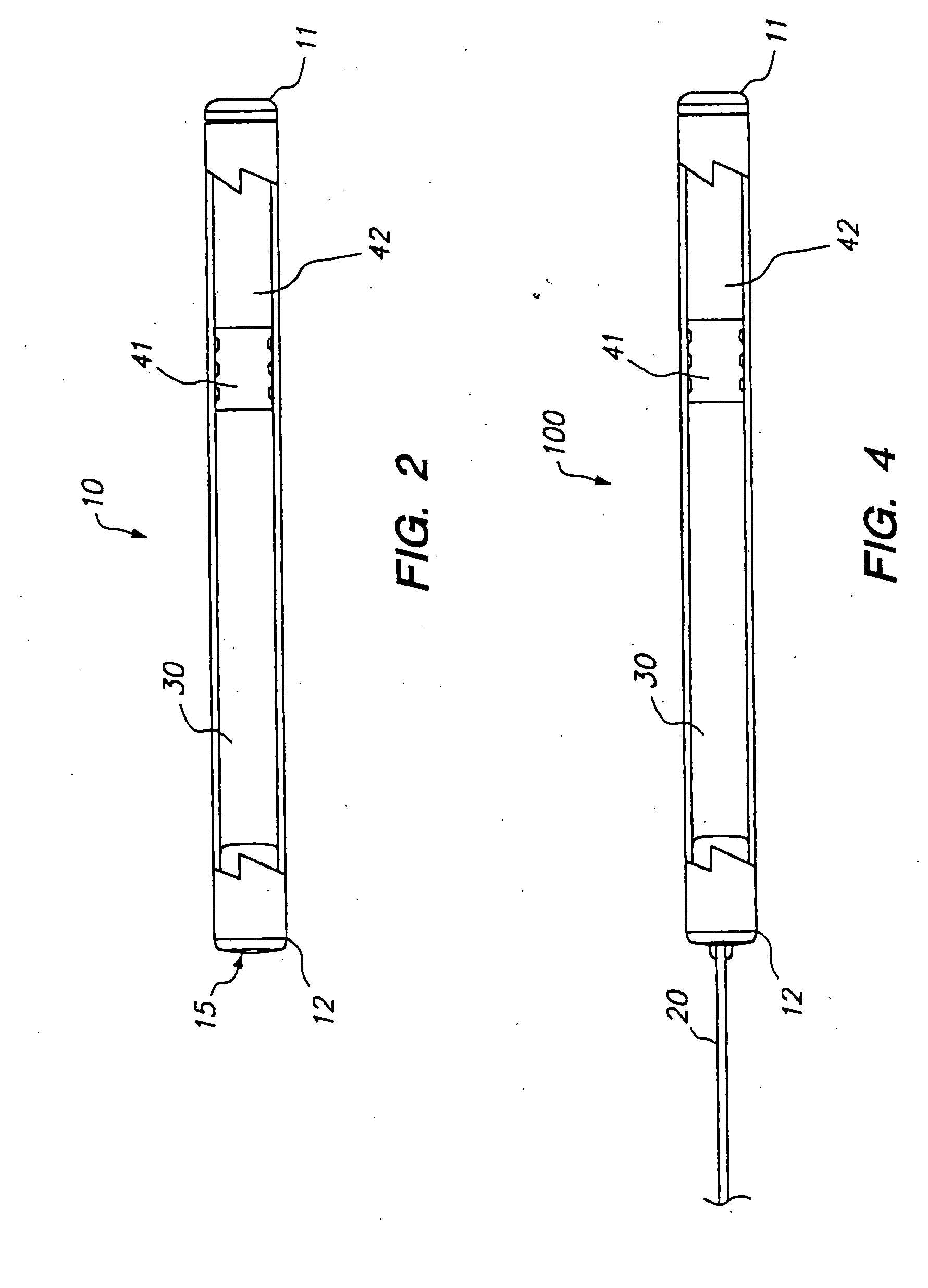
DUROS™ Pump Useful for Delivery of Sufentanil
[0119]The following is a description of exemplary drug loading parameters of a DUROS™ osmotic pump used for the delivery of sufentanil. The parameters are based on a nominal fill volume of the pump of 155 μl, with a nominal volumetric delivery rate of 1.4 μl / day for a nominal duration of 110 days (to ensure that a target delivery period of 90 days is achieved). An exemplary DUROS™ pump useful in this protocol is illustrated in FIG. 2, and is approximately 3.76 mm in diameter and 44.21 mm in length.
TABLE 1Loading ParametersmgDoseDosedeliveredNominalNominalDUROS ™wt / vol %raterateμg deliveredover 110RateRateResidenceformulationμg / hrμg / dayover 110 daysdaysμl / dayμl / hrVolumeload2.56066006.61.40.0581554.2851201080010.81.40.0581558.577.51801620016.21.40.05815512.6102402160021.61.40.05815517.1204604320043.21.40.05815534.3
These parameters thus dictate the amount of drug to be included in the formulation of the pump in order to provide for delivery ...
example 3
Formulations Comprising Sufentanil in Benzyl Alcohol
[0120]397 mg / mL Formulation
[0121]3.97 g of sufentanil base were weighed out and added to a portion of benzyl alcohol. The drug was dissolved in the benzyl alcohol by stirring with a magnetic stirrer. When the resultant preparation was clear, additional benzyl alcohol was added to obtain 10 mL of formulation. The resultant formulation concentration was 397 mg / mL.
[0122]310 mg / mL Formulation
[0123]3.1 g of sufentanil base were weighed out and added to a portion of benzyl alcohol. The drug was dissolved in the benzyl alcohol by stirring with a magnetic stirrer. When the resultant preparation was clear, additional benzyl alcohol was added to obtain 10 mL of formulation. The resultant formulation concentration was 310 mg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| octanol/water partition coefficient | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com