Neutralizing high affinity human monoclonal antibodies specific to rsv f-protein and methods for their manufacture and theraputic use thereof
a technology of rsv fprotein and monoclonal antibodies, which is applied in the direction of peptides, drug compositions, and infusion cells, can solve the problems of rsv infections that may be life-threatening, permanent lung damage or even life-threatening, and about 4500 deaths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Identification of Antibodies in Tumor Cell Cultures
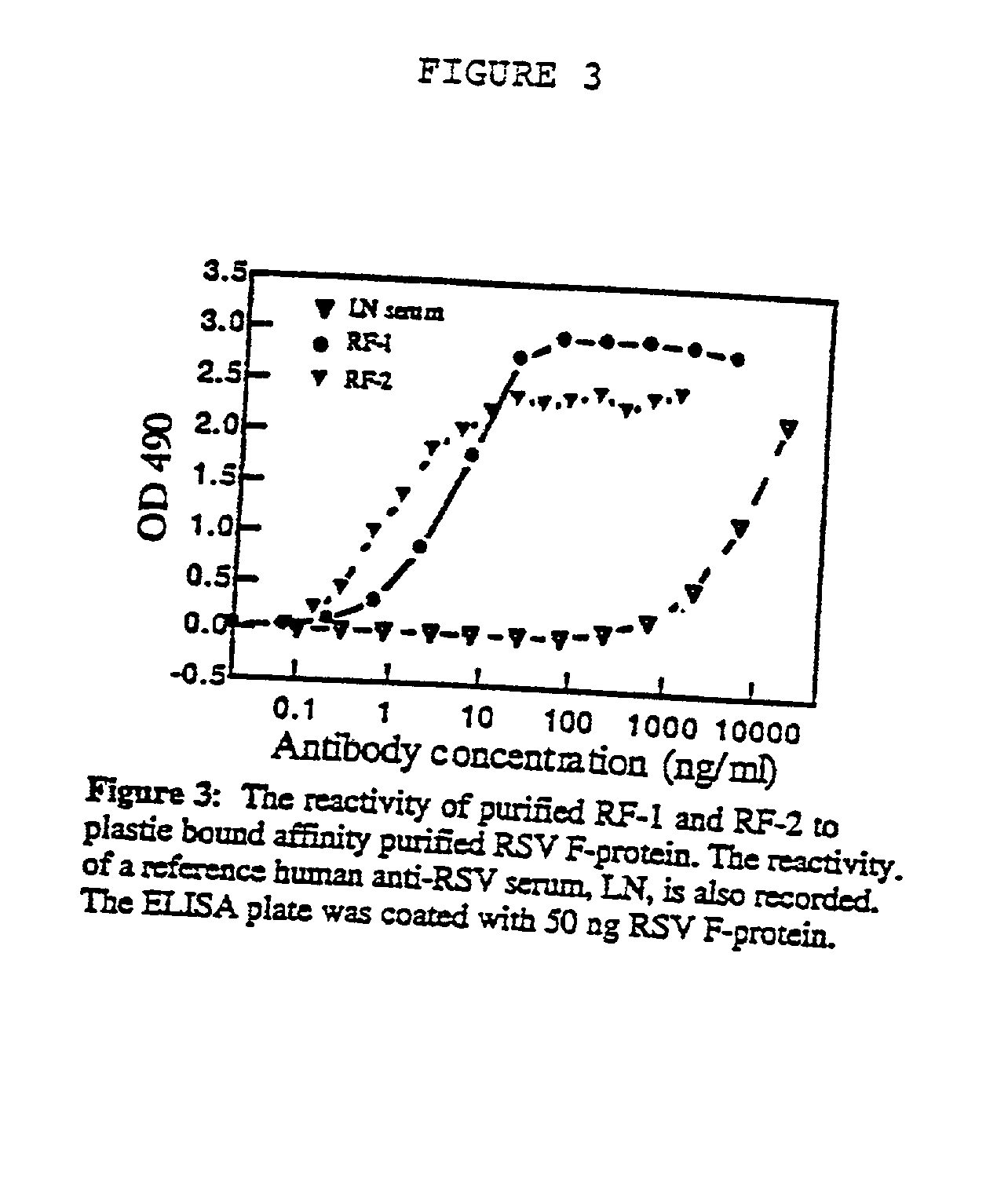
[0169] All mice with high anti-F protein titers were sacrificed and human cells were harvested from peritoneal lavage and spleens. Two mice (hu-SPL-SCID # 6 and hu-SPL-SCID #15) spontaneously developed abdominal solid tumors that were recovered and teased into single cell suspension. The tumor cells secreted specific anti-F protein antibodies as determined in ELISA. These tumors and antibodies are referred to as RF-1 CRSV F-protein) and RF-2. RF-1 and RF-2 were generated in two different experiments separated by approximately two months and were isolated from individual hu-SPL-SCID mice, and are thus distinct antibodies; they have established themselves in culture for more than 18 months and 16 months respectively, dividing with an approximate doubling time of 36-48 hours. Specific antibody concentration is typically of 0.5-1 .mu.g / ml in a culture seeded at 0.5.times.10.sup.6 cells / ml and grown for three days.
[0170] For further char...
example 3
Tissue Specificity of Anti-F-protein
[0171] Purified antibodies were screened for reactivity to a series of human cell lines available at ATCC by means of indirect immunofluorescence assays measured by flow cytometry (Table II): The results showed that the antibodies did not bind to cell lines representing respiratory tract lining (HEp-2, a laryngeal epidermoid carcinoma, Cat. No. CCL 23), liver (HepG2, a human hepatoma cell line, Cat. No. HB 8065), lymphoid tissue (SB, a human B lymphoblastoid cell line, Cat. No. CCL 120 and HSB, a T lymphoblastoid line, cat.no. CCL 120.1) and prostate (LNCaP.FGC, a human prostate adenocarcinoma line, Cat. No. CRL 1740).
example 4
[0172] To determine whether the antibodies had virus neutralizing effect in vitro, they were subjected to two types of functional assays: Infection neutralization assays were performed by pre-reacting the virus with purified MAb prior to its addition to the cells and therefore reflect the ability of the MAb to inhibit virus infectivity; fusion inhibition reflects the ability of the Ab to inhibit virus growth and expansion after virus entry in the cell. The outcome of both assays was measured as the amount of virus released in the culture after a given incubation time, as determined by viral antigen titration in EIA.
[0173] Both Abs were able to inhibit virus infection, of all twelve isolates tested, at concentrations ranging from 30 ng / ml to 1000 ng / ml and from 8 ng / ml to 165 ng / ml, for RF-1 and RF-2 respectively. RF-2 performed consistently better than RF-1, yielding to 50% virus inhibition (ED50) at concentrations 1.25 to 10 times lower than RF-1. Repres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com