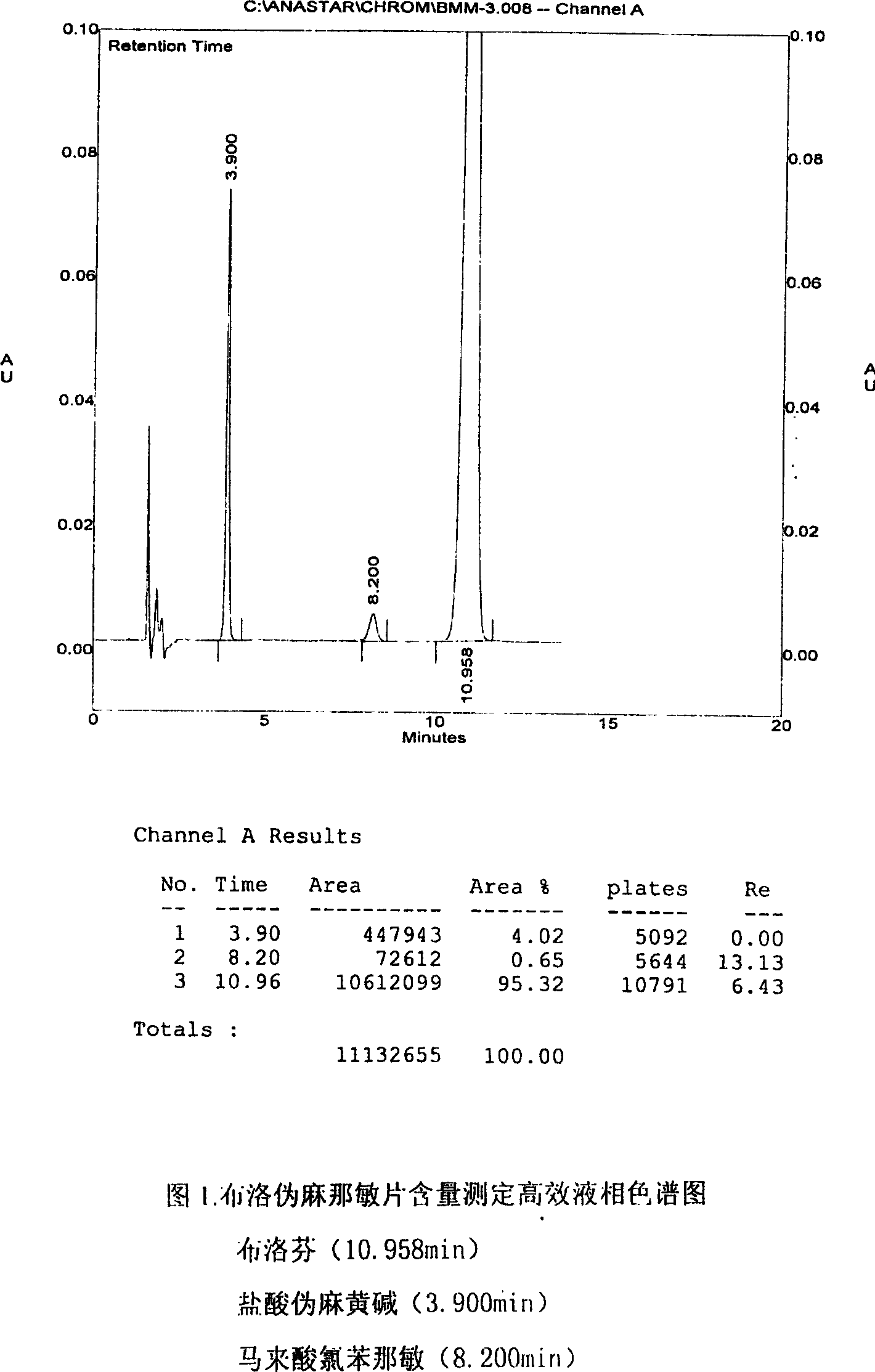
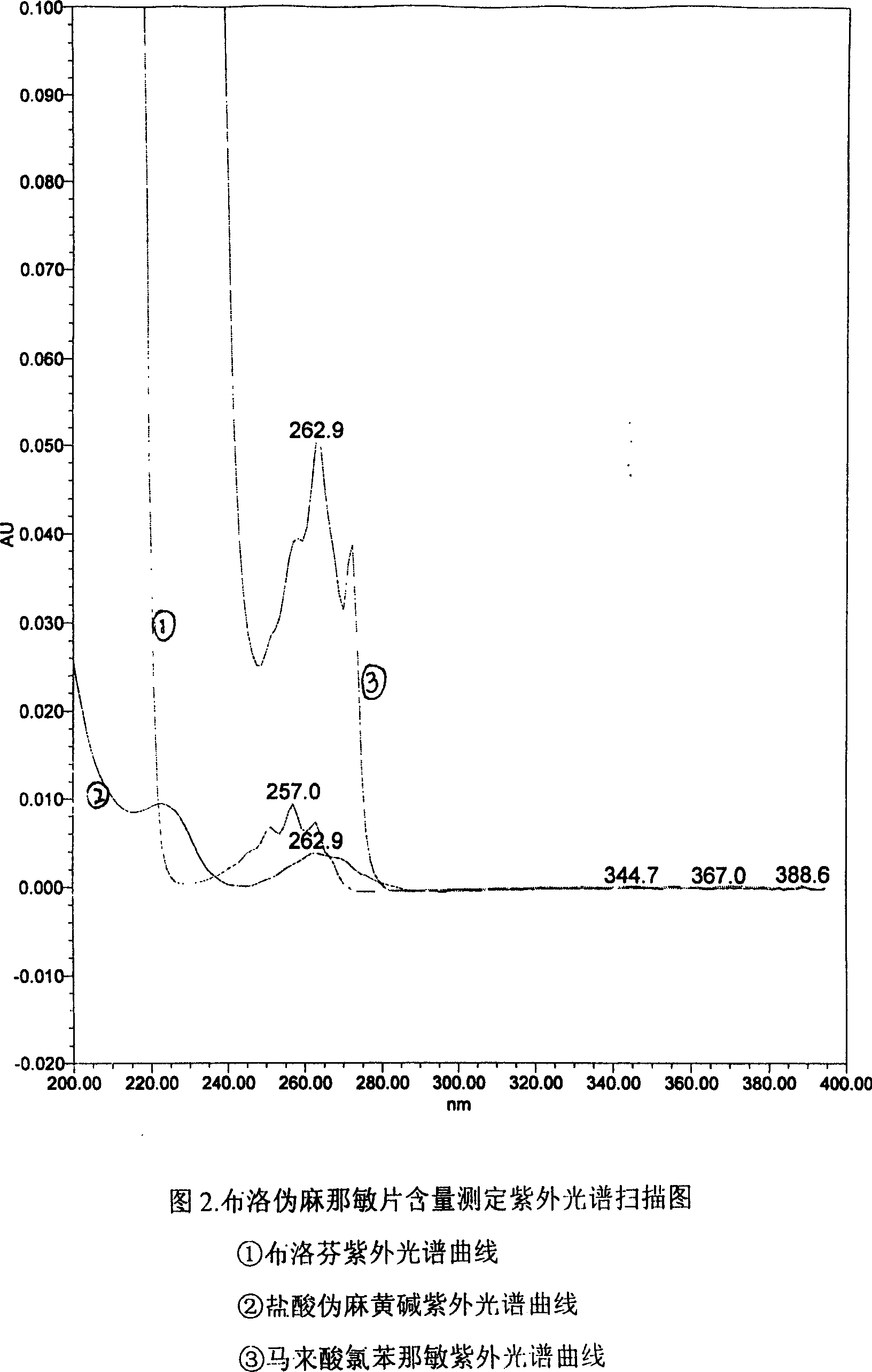
Content detecting method for Ibuprofen, chlorphenamine maleate and Pseudoephedrine Hydrochloride compound preparation
A detection method and a technology for compound preparations are applied in the field of content detection of ibuprofen-pseudoanamin compound preparations, which can solve the problems of high detection cost, long test time and the like, and achieve the effects of high sensitivity, good reproducibility and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Chromatographic conditions and system suitability test:
[0019] Waters 600 Pump type high performance liquid chromatograph (Waters, USA); Waters 996Photodiode Array detector (Waters, USA); Column: VP-ODS column (250mm×4.6mm; 100A); Mobile phase: potassium dihydrogen phosphate solution (8.16g potassium dihydrogen phosphate and 2.8g sodium lauryl sulfate in a 1000ml measuring flask, add water to dissolve, and dilute to the mark, shake well)-methanol-acetonitrile-triethylamine (40:20:40:0.1) It is the mobile phase (adjust the pH to 4.0-5.0 with phosphoric acid); the detection wavelength is 215nm-225nm; the number of theoretical plates should not be less than 3000 based on the ibuprofen peak. The resolution of each peak of ibuprofen, pseudoephedrine hydrochloride, and chlorpheniramine maleate should meet the requirements.
[0020] 2. Selection of detection wavelength:
[0021] Precisely weigh 20mg of chlorpheniramine maleate reference substance into a 50ml measuring bottle,...
Embodiment 2
[0026] Example 2: Determination of the content of ibuprofen and pseudoephedrine tablets
[0027] Take 20 ibuprofen and pseudoephedrine tablets, accurately weigh, grind, and accurately weigh an appropriate amount (about 40mg of ibuprofen) into a 100ml measuring flask, add phosphate buffer to the mark, dissolve it by ultrasound for 30 minutes, and filter After that, take the continued filtrate as the test solution; also take the reference substance ibuprofen 40mg, pseudoephedrine hydrochloride 6mg and chlorpheniramine maleate 0.4mg in the same 100ml measuring flask, add phosphate buffer solution to ultrasonic dissolve for 30 minutes, And dilute to the mark, shake up, as the reference solution; under the following selected chromatographic conditions: VP-ODS chromatographic column (250mm × 4.6mm; 100A); with potassium dihydrogen phosphate solution (8.16g potassium dihydrogen phosphate and Put 2.8g sodium lauryl sulfate in a 1000ml measuring flask, add water to dissolve, dilute to the ...
Embodiment 3
[0029] Example 3: Determination of the content of ibuprofen and pseudoephedrine capsules
[0030]Take 20 capsules of Buluo and Pseudoephedrine capsules. After precise weighing, pour out the contents (without losing the capsule shell), wipe the capsule shell with a small brush and weigh it accurately; mix the contents of the capsules evenly, grind finely, and weigh accurately Take an appropriate amount (approximately equivalent to 40mg of ibuprofen) and place it in a 100ml measuring flask, add phosphate buffer to the mark, dissolve it with ultrasound for 30 minutes, filter, and take the subsequent filtrate as the test solution; take the reference ibuprofen 40mg , Pseudoephedrine hydrochloride 6mg and chlorpheniramine maleate 0.4mg in the same 100ml measuring flask, add phosphate buffer solution to ultrasonic dissolve for 30 minutes, and dilute to the mark, shake well, as the reference solution; select the chromatogram below Condition: VP-ODS chromatographic column (250mm×4.6mm; 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com