Preparation method of sodium ferulate freeze dried powder injection
A technology for freeze-dried powder injection and sodium ferulate, which is applied in freeze-dried delivery, antidote, powder delivery, etc., and can solve the problem of high unqualified rate of clarity, poor product quality stability, and instability of sodium ferulate, etc. It can achieve good solubility, reduce vascular endothelial damage, and inhibit the synthesis of cholesterol.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
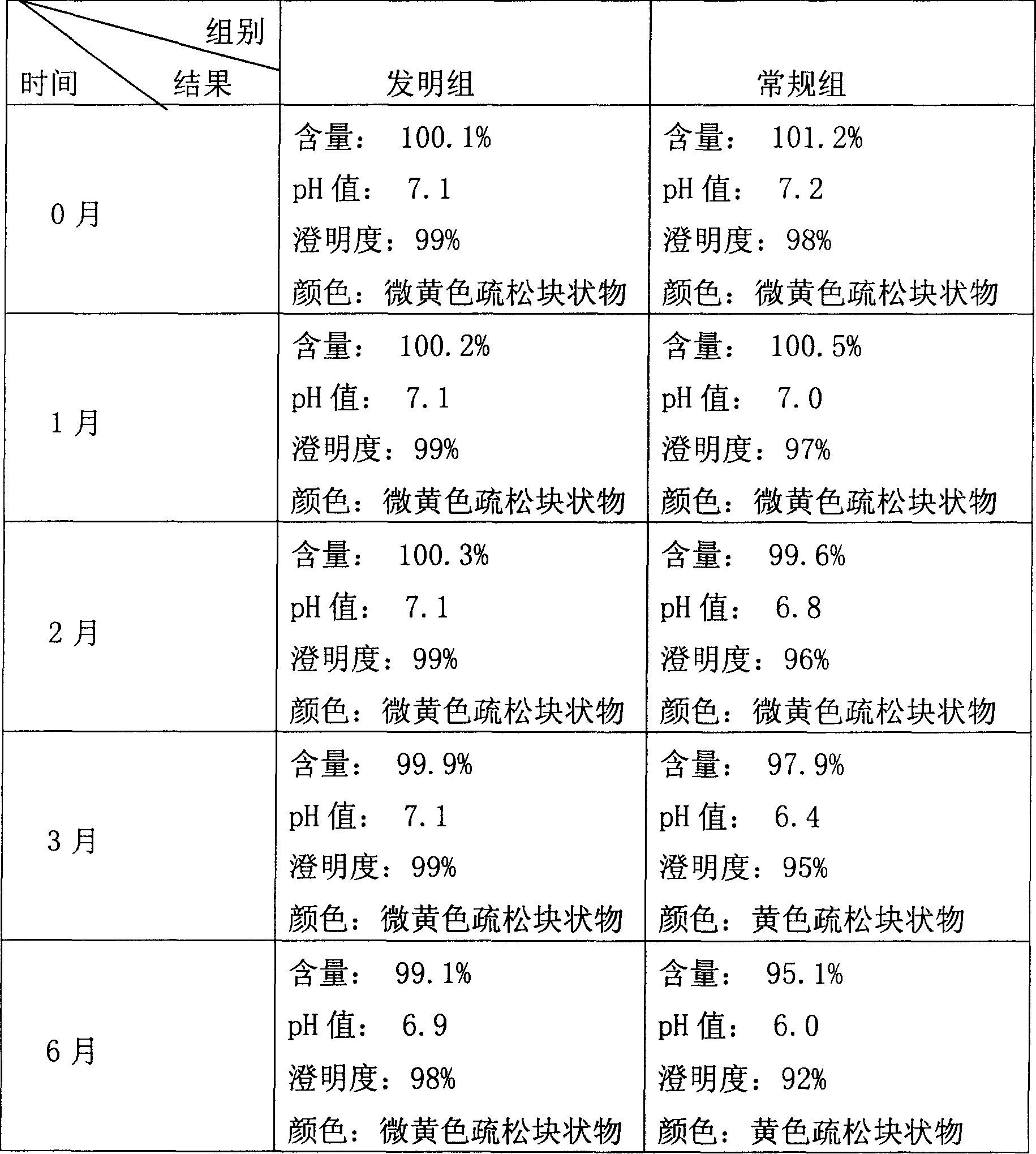
Examples
Embodiment 1
[0024] A) Take fresh water for injection with a preparation amount of 50%, cool it down to 40° C., feed nitrogen for 30 minutes, add sodium ferulate in the prescription in the dark, stir and dissolve it fully, and filter it with an ultrafiltration membrane with a molecular weight cut-off of 30,000. Filter to obtain an ultrafiltrate.
[0025] B) Preparation of mannitol filtrate: take 10% fresh water for injection, add mannitol in the prescription to dissolve, add 0.5% activated carbon, heat and boil for 15 minutes, circulate and cool the medicinal solution to 40°C, decarbonize and filter with a 0.45 μm filter membrane , and the filtrate is set aside.
[0026] C) Add the above-mentioned ultrafiltrate into the mannitol filtrate, mix evenly, constant volume, and adjust the pH value to 7.0. After the intermediate product is qualified, the liquid is sterilized and filtered through a terminal 0.22 μm filter membrane, and then filled with nitrogen under dark conditions. Filling in vi...
Embodiment 2
[0028] A) Take fresh water for injection with a preparation amount of 80%, cool it down to 20° C., feed nitrogen gas for 10 minutes, add sodium ferulate in the prescription in the dark, stir and dissolve it fully, and filter it with an ultrafiltration membrane with a molecular weight cut-off of 20,000. Filter to obtain an ultrafiltrate.
[0029] B) Preparation of mannitol filtrate: take 30% fresh water for injection, add mannitol in the prescription to dissolve, add 0.1% activated carbon, heat and boil for 30 minutes, circulate and cool the liquid medicine by 50°C, decarbonize and filter with a 0.45 μm filter membrane , and the filtrate is set aside.
[0030] C) Add the above-mentioned ultrafiltrate into the mannitol filtrate, mix evenly, constant volume, adjust the pH value to 7.2, after passing the inspection of the intermediate product, sterilize and filter the medicinal solution through a terminal 0.22 μm filter membrane, and fill it with nitrogen under dark conditions Fi...
Embodiment 3
[0032] A) Take fresh water for injection with a preparation amount of 60%, cool it down to 35° C., feed nitrogen gas for 25 minutes, add sodium ferulate in the prescription in the dark, stir and dissolve it fully, and filter it with an ultrafiltration membrane with a molecular weight cut-off of 10,000. Filter to obtain an ultrafiltrate.
[0033] B) Preparation of mannitol filtrate: take 15% fresh water for injection, add mannitol in the prescription to dissolve, add 0.4% activated carbon, heat and boil for 25 minutes, circulate and cool the medicinal solution to 46°C, decarbonize and filter with a 0.45 μm filter membrane , and the filtrate is set aside.
[0034] C) Add the above-mentioned ultrafiltrate into the mannitol filtrate, mix evenly, constant volume, adjust the pH value to 6.7, after passing the inspection of the intermediate product, sterilize and filter the medicinal solution through a terminal 0.22 μm filter membrane, and fill it with nitrogen under dark conditions ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com