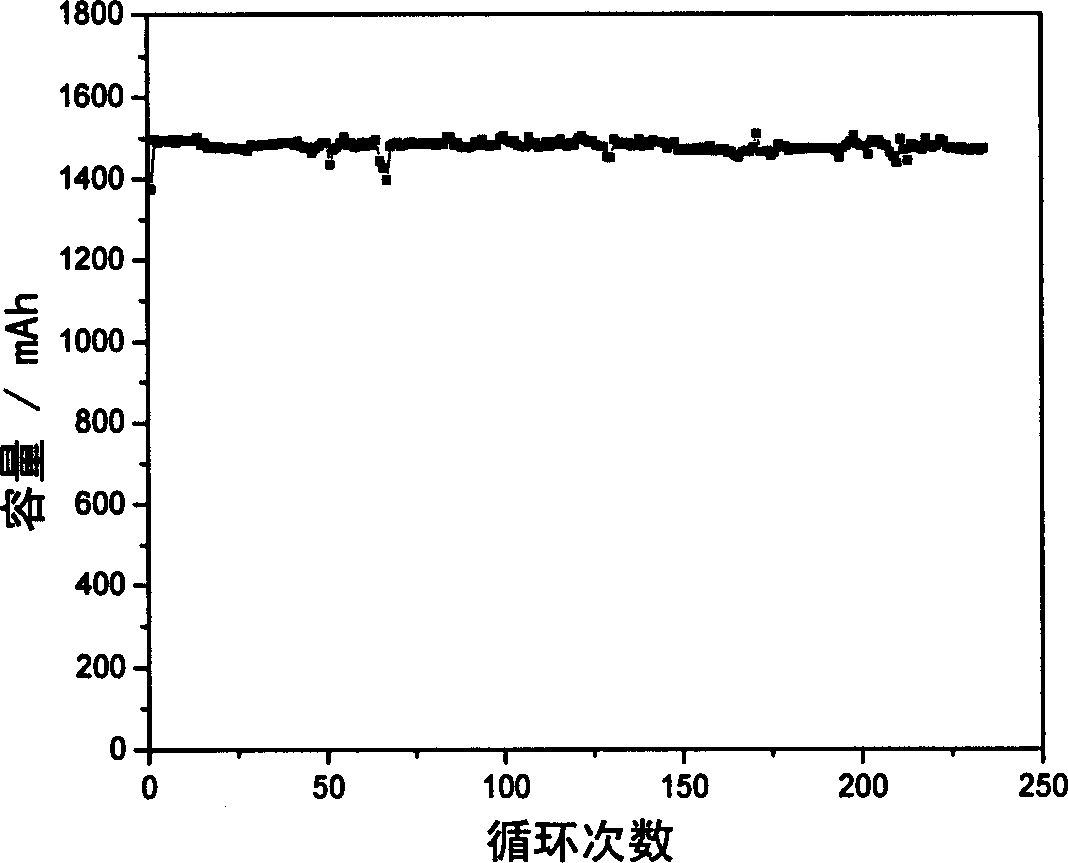
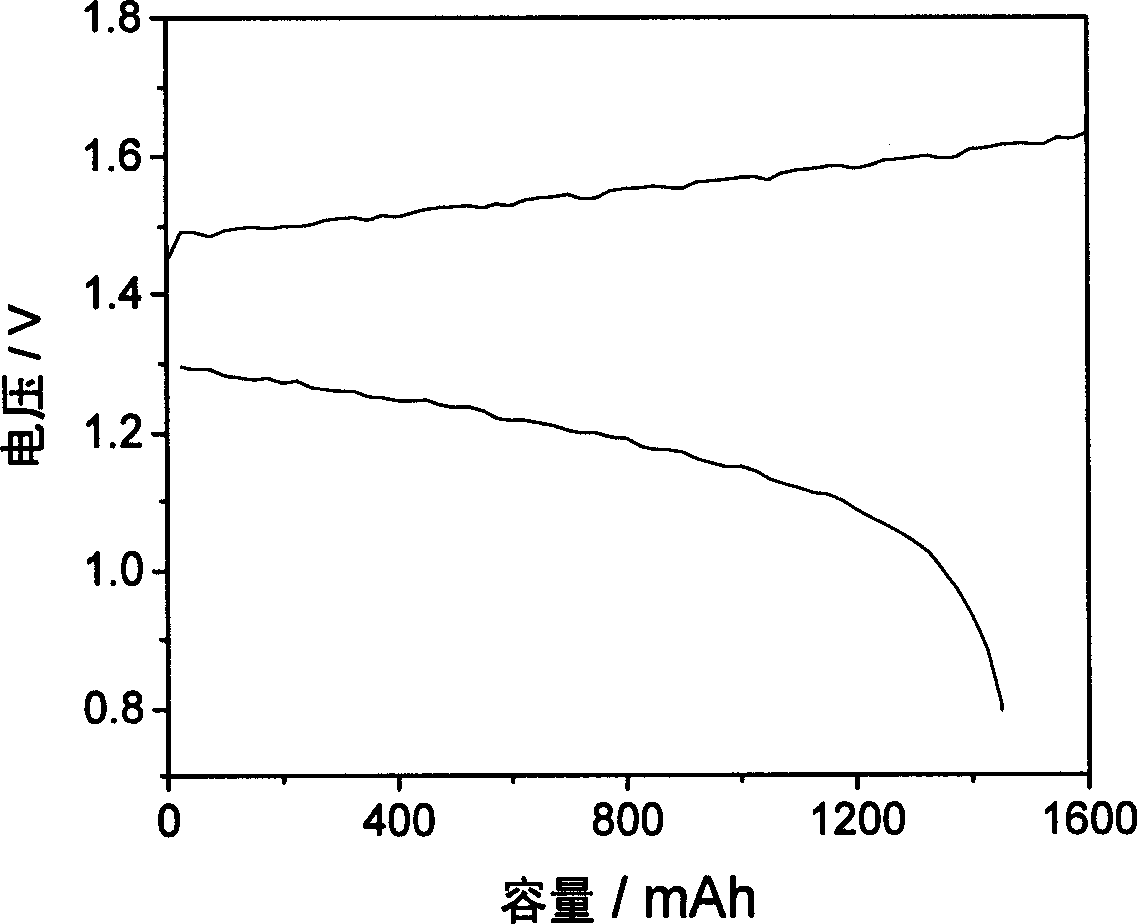
Proton exchange composite membrane for all vanadium redox flow battery and its preparing method
A proton exchange membrane, flow battery technology, applied in fuel cells, circuits, electrical components, etc., can solve the problems of poor vanadium ion barrier ability, unsuitable for wide application, poor chemical stability, etc., and achieve stable physical and chemical properties. , suitable for large-scale production, the effect of low film production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 30 g of polyvinylidene fluoride and 30 g of nano-silica powder in 500 ml of dimethylacetamide solvent. The mixed solution was heated and stirred in a water bath at 60° C. to obtain a viscous solution. This viscous solution was cast on a glass plate and dried at 60°C to form a film, which was dried and rinsed in deionized water. Thereafter, the membrane was immersed in a styrene sulfonic acid solution at 80° C. for 5 hours. Take out the membrane and wash the surface with deionized water to obtain a composite membrane of polyvinylidene fluoride
[0022] Effect: at room temperature, the conductivity is 1.02×10 -2 S / cm.
Embodiment 2
[0024] Dissolve 25 g of polyvinylidene fluoride and 5 g of molecular sieve powder in 100 ml of dimethyl sulfoxide solvent. The mixed solution was heated and stirred in a water bath at 80° C. to obtain a viscous solution. This viscous solution was cast on a glass plate and dried at 150°C to form a film, which was dried and rinsed in deionized water. Thereafter, the membrane was immersed in 2-acrylamide-2-methylpropanesulfonic acid solution at 70° C. for 8 hours. Take out the membrane and wash the surface with deionized water to obtain a composite membrane of polyvinylidene fluoride
[0025] Effect: at room temperature, the conductivity is 2.04×10 -2 S / cm.
Embodiment 3
[0027] Dissolve 30 g of polyvinylidene fluoride and 3 g of nano-alumina powder in 200 ml of propylene carbonate solvent. The mixed solution was heated and stirred in a water bath at 50° C. to obtain a viscous solution. This viscous solution was cast on a glass plate and dried at 120°C to form a film, which was dried and rinsed in deionized water. Thereafter, the membrane was immersed in vinylsulfonic acid solution at 60° C. for 16 hours. Take out the membrane and wash the surface with deionized water to obtain a composite membrane of polyvinylidene fluoride
[0028] Effect: at room temperature, the conductivity is 3.05×10 -2 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com