Recombinant nucleic acid useful for inducing protective immune response against allergens
A recombinant nucleic acid and allergen technology, applied in allergen antigen components, recombinant DNA technology, vaccines, etc., can solve problems such as inappropriate allergens and weak immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0091] Materials and Methods
[0092]Animals and Immunization: Six to eight week old female BALB / cJ mice were purchased from the Experimental Animal Centre, Lorong Chencharu, Sembawang, Singapore. Animals were housed in conventional animal housing at the NUS Animal Conservation Unit. For immunization with uncoated DNA, animals were injected im or subcutaneously with 100 μg of plasmid per dose (2 injections of 50 μl each) on days 0, 7 (for Blot5 only with minigene injection) and 14 days. On day 21 and on day 42 or 49, the animals were administered intraperitoneally with an appropriate dose of alum-adsorbed mite allergen (Blo t5 or Der p1). For some specific experiments, animals were further exposed to a yeast recombinant dust mite allergen spray (0.5 mg per ml PBS) on days 63, 66 and 69. Sera were collected weekly and stored at -20°C until assayed. The titer and type of dust mite allergen-specific antiserum were determined by ELISA test. Inhalation challenges were performed...
Embodiment 1
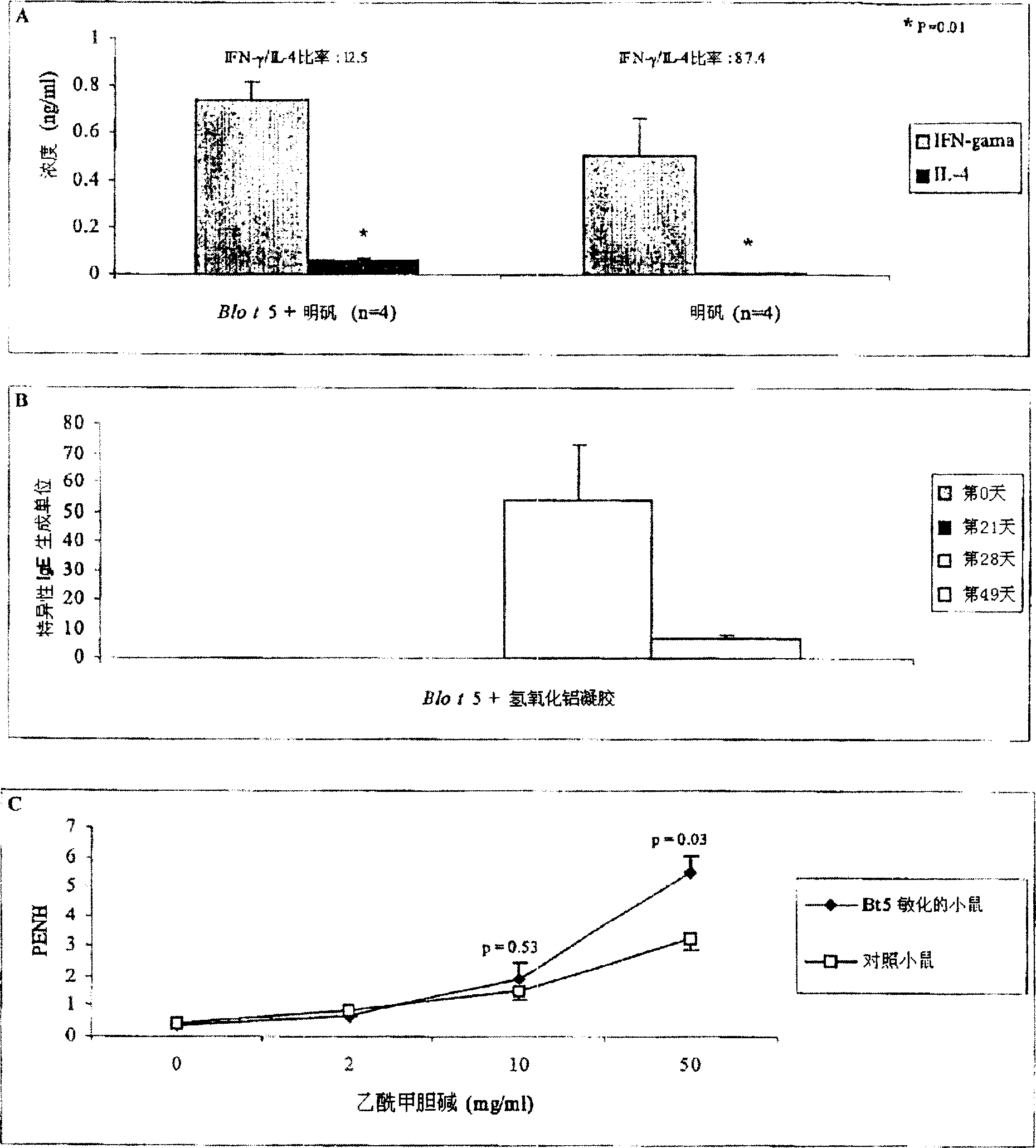
[0204] Six- to eight-week-old animals (n=4 per group) were intraperitoneally administered 10 μg and 5 μg of yeast recombinant Blo t5 in 4 mg alum (Aluminum Hydroxide Gel R) on days 0 and 21, respectively. Serum was collected weekly and stored at -20°C until ELISA assay. The level of Blo t5-specific IgE antiserum was determined by ELISA assay. One antibody producing unit is equivalent to one nanogram of mouse Ig per ml of serum ( figure 1 A). On day 21, spleen single-cell suspensions were prepared from mice predosed with alum-adsorbed Blo t5 or alum alone (day 0). Splenocytes were stimulated with Bt550-67 peptide (5 μM) for 72 hours. The levels of IFNγ and IL-4 in the culture supernatant were determined by ELISA assay ( figure 1 B). Six- to eight-week-old animals (n=3 or 4 per group) were intraperitoneally administered 2 mg of alum containing 10 μg and 5 μg of yeast recombinant Blo t5 on days 0 and 21, respectively. On days 28, 31 and 34, these animals were further booste...
Embodiment 2
[0207]Six to eight week old animals (n=4 per group) were injected intramuscularly with 100 μg of pCI-Blo t5 and pCI on days 0 and 14. These animals were then dosed twice intraperitoneally with 4 mg alum containing 10 μg (day 21) and 5 μg (day 42) of yeast recombinant Blo t5 allergen. Sera were collected weekly and stored at -20°C until assayed. Determination of Blo t5 specific IgG by ELISA assay 2a ( figure 2 A) and IgE ( figure 2 B) Levels of antiserum. One antibody producing unit is equivalent to one nanogram of mouse Ig per ml of serum.
[0208] Immune responses of animals receiving muscle immunization without coat gene and alum-adsorbed Blo t5 enhancer as shown in figure 2 shown. As shown, whole-gene immunization with Blo t5 was able to generate a Th1-dominant immune response in animals receiving three intramuscular injections of pCI-Blot5, with Blo t5-specific serum IgG seen as early as day 21 2a There was a significant increase in the level of ( figure 2 A). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com