Compound of dual functional esterified prodrug of Cefetamet, and oral preparation
The technology of a pharmaceutical compound, ceftazidime, is applied in the field of esterified prodrug compounds and their oral preparations, which can solve the problems of low solubility, bitter taste, and low bioavailability, and achieve improved water solubility, increased sweetness, and expanded applications range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
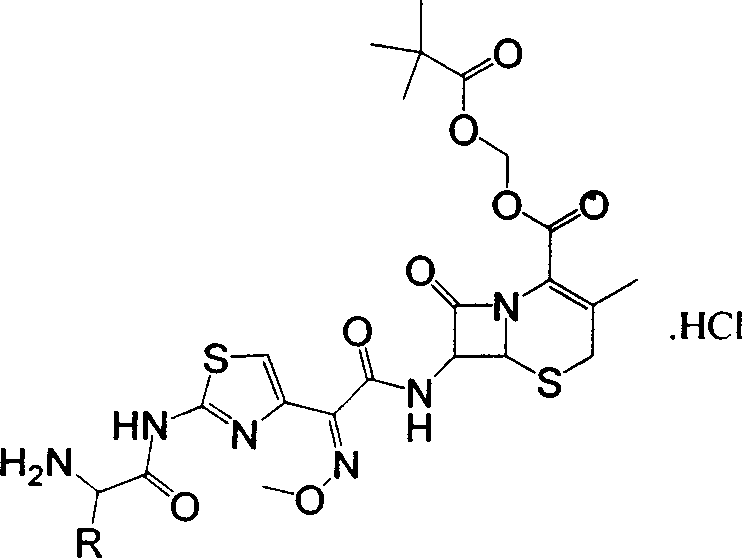
[0026] Example 1: (6R, 7R)-3-methyl-7-[(2-(N-tert-butoxycarbonyl-(S)-alanylamino-4-thiazolyl)-(methoxyimino )Acetamido)]-8-oxo-5-thia-1-azabicyclo[4,2,0]-2-ene-2-carboxylic acid trimethylacetoxymethyl ester (a 1 ) preparation
[0027] Add 19.2 g of ceftazidime pivoxil and 15 g of N-tert-butoxycarbonyl-L-albamate into 200 ml of dichloromethane, stir at room temperature for 10 minutes, then add 1-ethyl-3-(3-dimethyl Aminopropyl) carbodiimide hydrochloride 15.2g, 4-dimethylaminopyridine 0.44g, stirred and reacted at a temperature of 18-25°C for 2 hours, added 10% citric acid solution 60ml to wash once, 5% NaHCO 3 Wash once with 60ml solution, once with saturated NaCl solution, take the organic phase and add anhydrous MgSO 4 1 g was dried for 30 minutes, filtered, and concentrated to dryness at 40°C to obtain a 1 , 19.4g, content 97.5%, yield 75.7%. C 29 h 40 N 6 o 10 S 2 The elemental analysis values are C, 49.99; H, 5.79; N, 12.06; S, 9.20. Actual measured values: C...
Embodiment 2
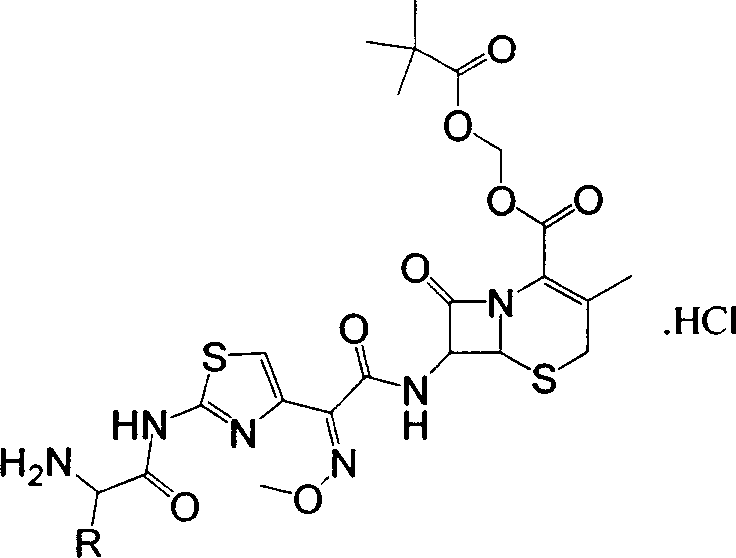
[0028] Example 2: (6R,7R)-3-methyl-7-[(2-(s)-alanylamino-4-thiazolyl)-(methoxyimino)acetamido]-8-oxo -5-Thia-1-azabicyclo[4,2,0]-2-ene-2-carboxylic acid trimethylacetoxymethyl ester hydrochloride (b 1 ) preparation
[0029] In the reaction bottle, put a 1 Dissolve 11g in 55ml of formic acid, control the temperature at 10-15°C, add 11ml of ethanol, dropwise add 1.8ml of concentrated HCl, stir for 10 minutes, add 110ml of isopropanol to precipitate a large amount of solid, cool down to 0-5°C, stir and crystallize for 2 hours, Filter, wash twice with isopropanol, and dry under vacuum at 50°C to obtain a powdery off-white solid b 1 , 8.6g, content 98.0%, yield 86.2%. C 23 h 31 ClN 6 o 8 S 2 The elemental analysis values are C, 44.62; H, 5.05; Cl, 5.73; N, 13.57; S, 10.36. Actual measured values: C, 44.4; H, 5.12; Cl, 5.83; N, 13.49; S, 10.31.
Embodiment 3
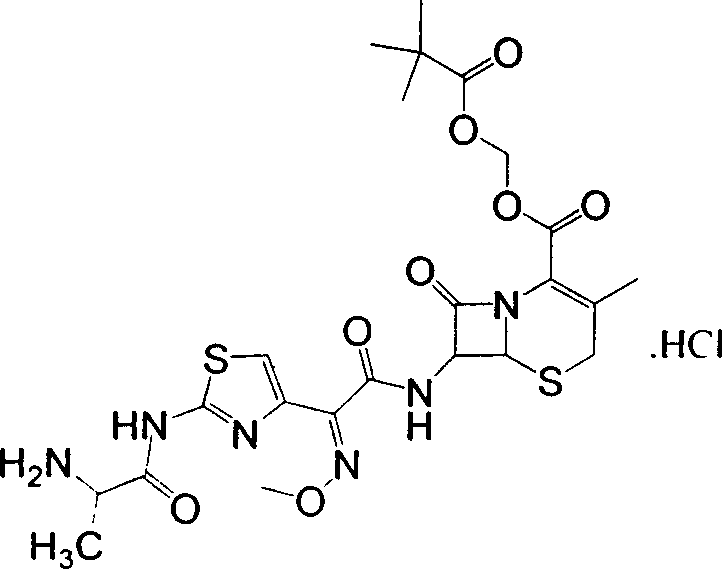
[0030] Example 3: (6R,7R)-3-methyl-7-[(2-(N-tert-butoxycarbonyl-glycylamino-4-thiazolyl)-(methoxyimino)acetamido)] -8-Oxo-5-thia-1-azabicyclo[4,2,0]-2-ene-2-carboxylic acid trimethylacetoxymethyl ester (a 2 ) preparation
[0031] Add 19.2 g of ceftazidime pivoxil and 6.6 g of N-tert-butoxycarbonylglycine into 200 ml of dichloromethane, stir at room temperature for 10 minutes, add 1-ethyl-3-(3-dimethylaminopropyl) carbon Diimine hydrochloride 15.2g, 4-dimethylaminopyridine 0.44g, stirred and reacted at a temperature of 18-25°C for 2 hours, then added 60ml of 10% citric acid solution to wash once, 5% NaHCO 3 Wash once with 60ml solution, once with saturated NaCl solution, take the organic phase and add anhydrous MgSO 4 1 g was dried for 30 minutes, filtered, and concentrated to dryness at 40°C to obtain a 2 , 15.7g, yield 62.6%. C 28 h 38 N 6 o 10 S 2 Elemental analysis values: C, 49.26; H, 5.61; N, 12.31; S, 9.39. The actual measured values are: C, 49.15; H, 5.72; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com