Medicinal composition containing tanshinon II A sodium sulfonate and its quality control method
A technology of tanshinone and sodium sulfonate, applied in the field of medicine, can solve the problems of inability, high polarity, and difficult retention of octadecylsilane-bonded silica gel column, and achieves strong controllability, high accuracy and reproducibility. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
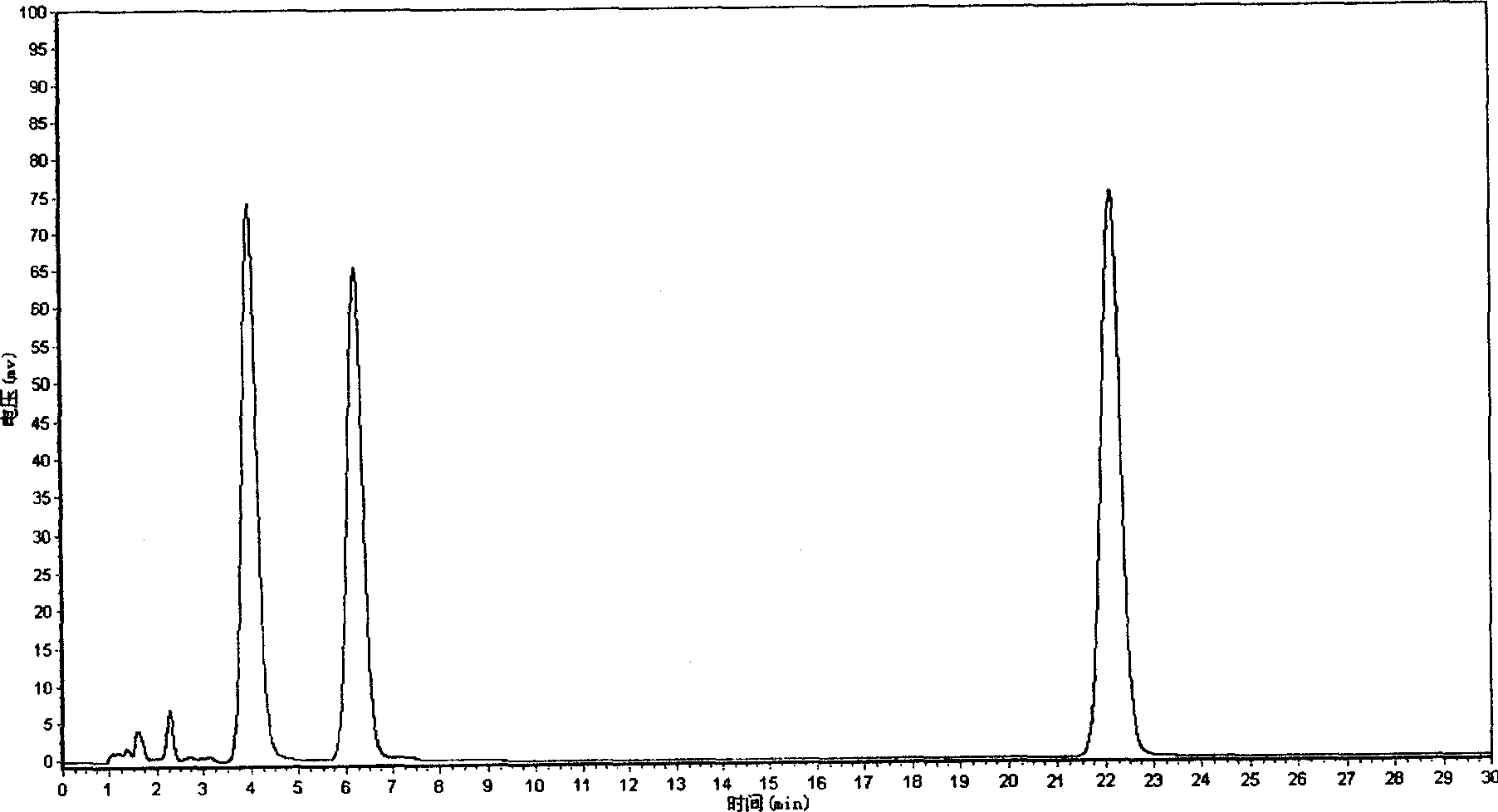
[0071] Example 1 Related substance inspection and content determination of Tanshinone IIA sodium sulfonate bulk drug
[0072] relative substance
[0073]Determined by high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition two appendix V D), using octadecylsilane bonded silica gel as filler, methanol-water (volume ratio 70:30) (every 100mL water contains tetrabutyl hydroxide Ammonium (2mmol) was used as the mobile phase, the flow rate was 1mL / min, and the detection wavelength was 246nm. Get an appropriate amount of tanshinone I sulfonate sodium reference substance (self-made, confirmed by structure), accurately weighed, add mobile phase to make a solution containing 20 μg of tanshinone I sulfonate sodium in every 1 mL, and accurately measure the tanshinone I sulfonate sodium reference substance solution 10 μL was injected into the chromatograph, the chromatogram was recorded, and the peak area was calculated. Another take tanshinone IIA sodium sulfonate cr...
Embodiment 2
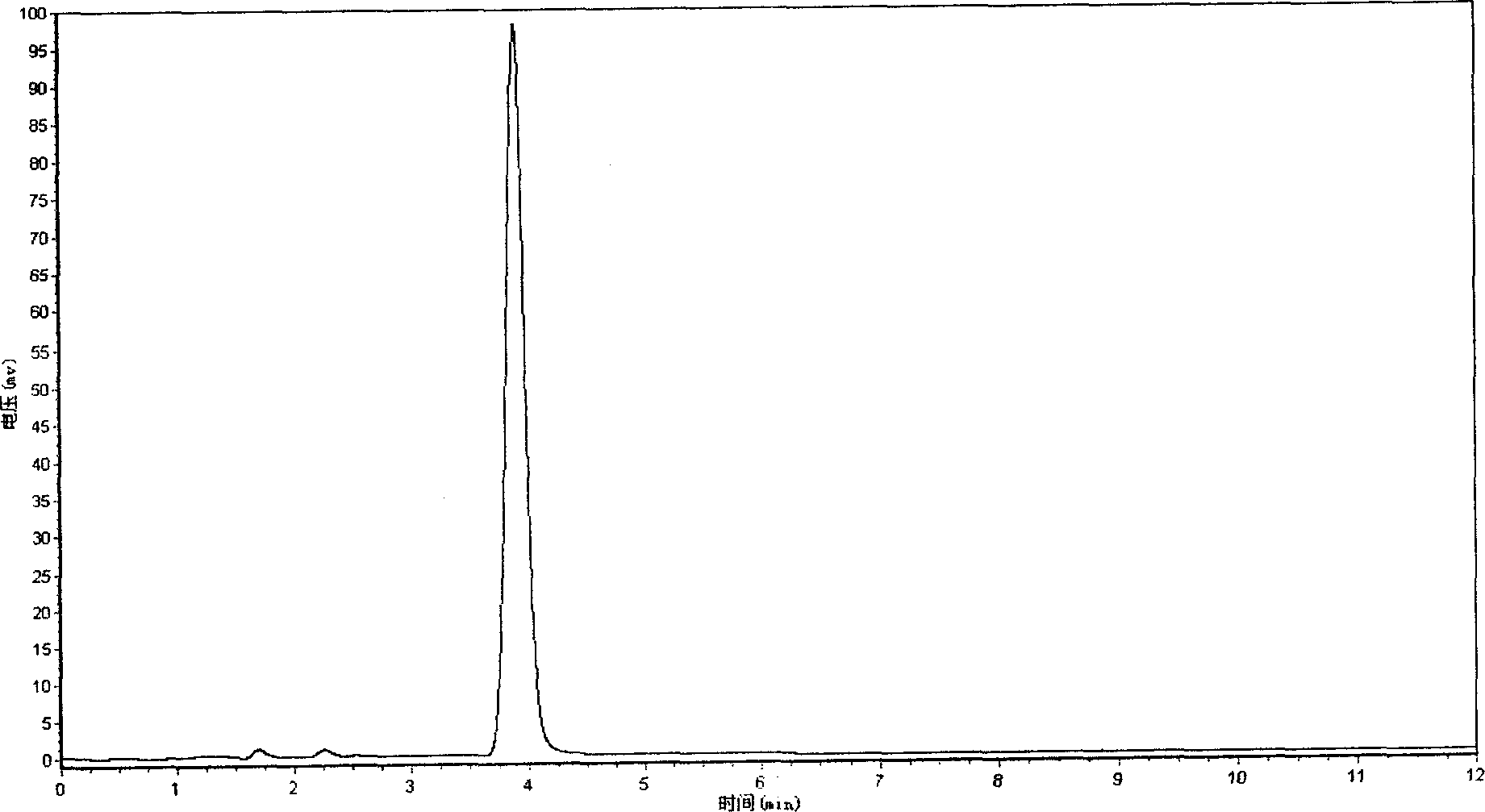
[0079] Example 2 Related substance inspection and content determination of tanshinone IIA sodium sulfonate bulk drug
[0080] relative substance
[0081] Determined by high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition two appendix V D), with octadecylsilane bonded silica gel as filler, methanol-water (volume ratio 75:25) (every 100mL of water containing tetrabutyl hydroxide Ammonium (2mmol) was used as the mobile phase, the flow rate was 1mL / min, and the detection wavelength was 250nm. Take an appropriate amount of tanshinone I sulfonate reference substance (self-made, confirmed by structure) and tanshinone IIA reference substance, accurately weighed, add dichloromethane and methanol to dissolve, add mobile phase to make each 1mL containing tanshinone I sulfonate sodium 20 μg and Tanshinone IIA 20μg mixed reference substance solution, accurately measure 10μL of mixed reference substance solution and inject it into the chromatograph, record the chromat...
Embodiment 3
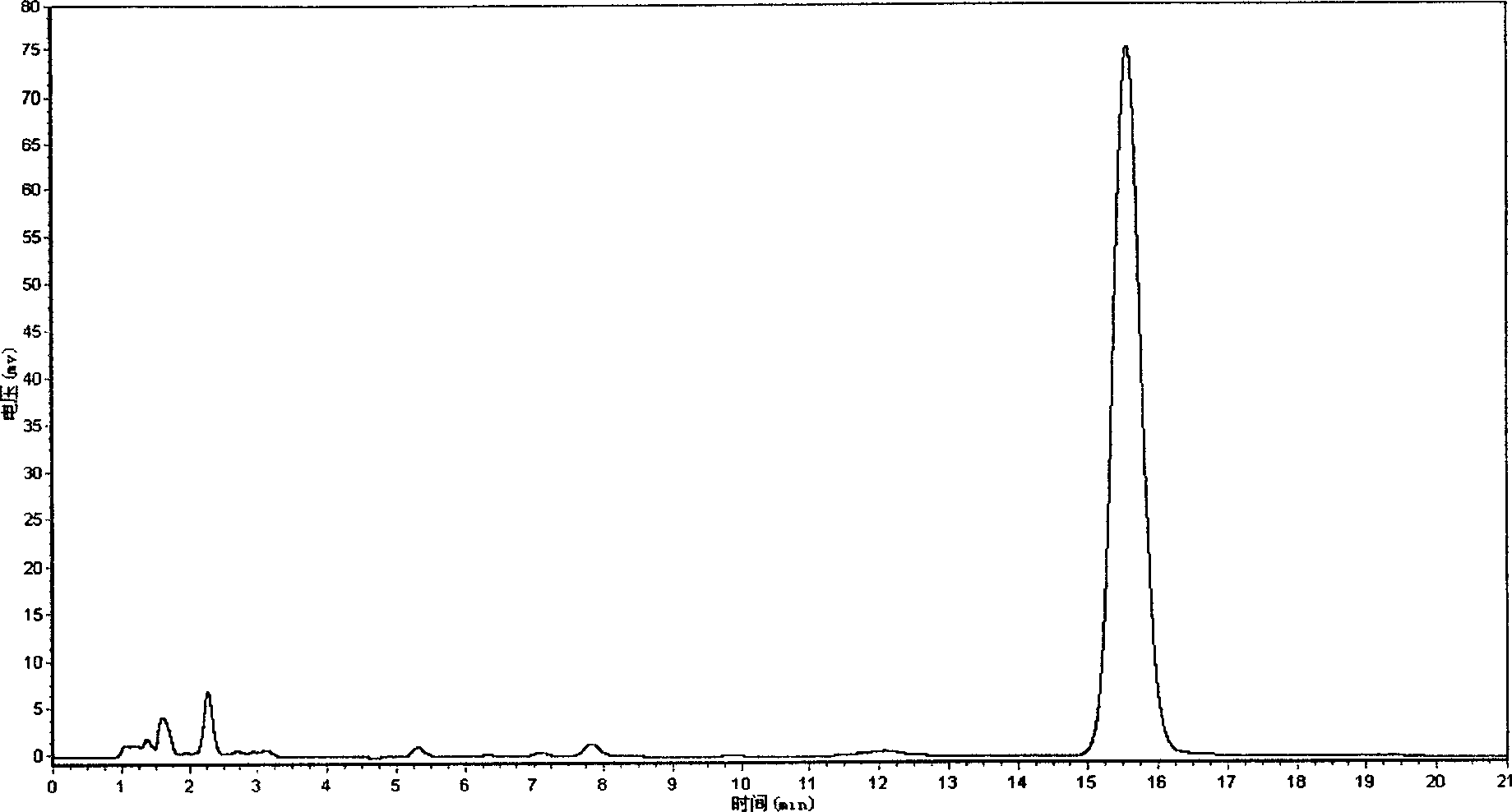
[0083] Example 3 Inspection and Content Determination of Related Substances of Tanshinone IIA Sodium Sulfonate Powder Injection
[0084] relative substance
[0085] Measured by high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition two appendix V D), with octadecylsilane bonded silica gel as filler, with acetonitrile-water (volume ratio 50:50) (every 100mL water contains tetramethyl Ammonium bisulfate (0.2mmol) was the mobile phase, the flow rate was 2mL / min, and the detection wavelength was 246nm. Get an appropriate amount of tanshinone I sulfonate sodium reference substance (self-made, confirmed by structure), accurately weighed, add mobile phase to make a solution containing 20 μg of tanshinone I sulfonate sodium in every 1 mL, and accurately measure the tanshinone I sulfonate sodium reference substance solution 10 μL was injected into the chromatograph, the chromatogram was recorded, and the peak area was calculated. Take another tanshinone IIA sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com