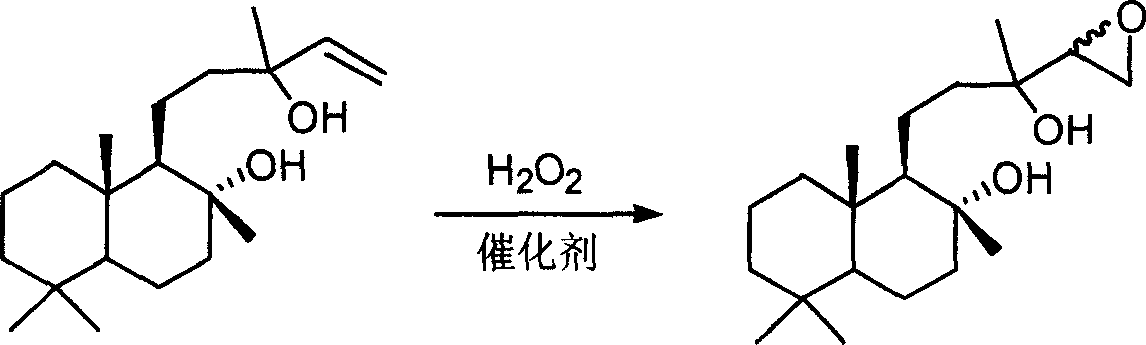
Synthetic method for epoxy sclareol
A synthesis method and technology of sclareol, applied in the direction of organic chemistry and the like, can solve the problems of high cost, low atom utilization rate, poor stability of peroxyacid, etc., and achieve the effect of low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 30.8g of sclareol in 90mL of 1,2-dichloroethane, add 1.5g of 12-phosphotungstic acid, 0.72g of cetylpyridinium chloride and 35mL of 30% aqueous hydrogen peroxide to adjust the pH to ≈2.0 , stirred vigorously at 70°C for 12 hours. The obtained reaction mixture was separated and purified according to conventional methods to obtain 30.4 g of epoxy sclareol as a white solid, with a yield of 93.8%. The melting point is 131-132°C.
Embodiment 2
[0028] Dissolve 30.8g of sclareol in 60mL of chloroform, and add 0.41g of sodium tungstate dihydrate, 0.72g of sodium dihydrogen phosphate, 0.36g of cetylpyridinium chloride and 90mL of 10% hydrogen peroxide solution to adjust the pH to ≈1.5 , stirred vigorously at 70°C for 24 hours. The resulting reaction mixture was separated and purified by conventional methods to obtain 28.4 g of epoxy sclareol as a white solid, with a yield of 87.8%. The melting point is 130-132°C.
Embodiment 3
[0030] Dissolve 30.8g of sclareol in 60mL of ethyl acetate, add 2.0g of hexadecyl phosphotungstate pyridinium salt and 21mL of 50% hydrogen peroxide aqueous solution, and react vigorously at 70°C for 8 hours. The resulting reaction mixture was separated and purified by conventional methods to obtain 30.2 g of epoxyslareol as a white solid, with a yield of 93.2%. The melting point is 130-132°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com